Vaccine approaches conferring cross-protection against influenza viruses
- PMID: 28925296
- PMCID: PMC6005355
- DOI: 10.1080/14760584.2017.1379396
Vaccine approaches conferring cross-protection against influenza viruses
Abstract
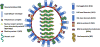
Annual vaccination is one of the most efficient and cost-effective strategies to prevent and control influenza epidemics. Most of the currently available influenza vaccines are strong inducers of antibody responses against viral surface proteins, hemagglutinin (HA) and neuraminidase (NA), but are poor inducers of cell-mediated immune responses against conserved internal proteins. Moreover, due to the high variability of viral surface proteins because of antigenic drift or antigenic shift, many of the currently licensed vaccines confer little or no protection against drift or shift variants. Areas covered: Next generation influenza vaccines that can induce humoral immune responses to receptor-binding epitopes as well as broadly neutralizing conserved epitopes, and cell-mediated immune responses against highly conserved internal proteins would be effective against variant viruses as well as a novel pandemic influenza until circulating strain-specific vaccines become available. Here we discuss vaccine approaches that have the potential to provide broad spectrum protection against influenza viruses. Expert commentary: Based on current progress in defining cross-protective influenza immunity, it seems that the development of a universal influenza vaccine is feasible. It would revolutionize the strategy for influenza pandemic preparedness, and significantly impact the shelf-life and protection efficacy of seasonal influenza vaccines.
Keywords: Influenza viruses; avian influenza viruses; cross protection; novel influenza vaccines; pandemic influenza; pandemic preparedness; universal influenza vaccine.
Figures


References
-
- WHO. WHO. World Health Organization; 2017. Influenza (Seasonal) (Ed.^(Eds)
-
- Thompson MG, Li DK, Shifflett P, et al. Effectiveness of seasonal trivalent influenza vaccine for preventing influenza virus illness among pregnant women: a population-based case-control study during the 2010–2011 and 2011–2012 influenza seasons. Clin Infect Dis. 2014;58(4):449–457. - PubMed
-
- FAO H7N9 situation update - Avian Influenza A(H7N9) virus - FAO Emergency Prevention System for Animal Health (EMPRES-AH) 2017 (Ed.^(Eds)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
