Functional significance and therapeutic implication of ring-type E3 ligases in colorectal cancer
- PMID: 28925398
- PMCID: PMC5770599
- DOI: 10.1038/onc.2017.313
Functional significance and therapeutic implication of ring-type E3 ligases in colorectal cancer
Abstract
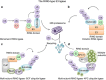
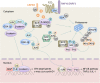
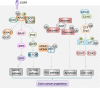
Accumulative studies revealed that E3 ubiquitin ligases have important roles in colorectal carcinogenesis. The pathogenic mechanisms of colorectal cancer (CRC) initiation and progression are complex and heterogeneous, involving somatic mutations, abnormal gene fusion, deletion or amplification and epigenetic alteration, which may cause aberrant expression or altered function of E3 ligases in CRC. Defects of E3 ligases have been reported to be involved in the molecular etiology and pathogenesis of CRC. The aberrant expressed E3 ligases can function as either oncogenes or tumor suppressors depending on ubiquiting target substrates in CRC. Recently, considerable progress has been made in our understanding of the potential roles of E3 ligase-mediated ubiquitylation in colorectal carcinogenesis. There are mainly two subtypes of E3 ubiquitin ligases in humans, as defined by the presence of either a HECT domain or a RING finger domain on the basis of structural similitude. Most cancer-associated E3 ligases participate in regulating the cell cycle, apoptosis, gene transcription, cell signaling and DNA repair, the critical parts of CRC tumorigenesis. In this review, we have provided a comprehensive summary of abnormally expressed E3 ligases and their related pivotal mechanistic effects in CRC. In particular, we have highlighted the function of RING-type E3 ubiquitin enzymes in modulating cancer signaling pathways, immunity and tumor microenvironment in CRC development and progression; their mechanism(s) of action in CRC involving both ubiquitylation-dependent and ubiquitylation-independent effects; and the potential of RING E3 ligases as molecular biomarkers for predicting patient prognosis and as therapeutic targets in CRC. A better understanding of E3 ligase-mediated substrates' ubiquitylation involved in the development of CRC will provide new insights into the pathophysiology mechanisms of CRC, and unravel novel prognostic markers and therapeutic strategies for CRC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. - PubMed
-
- Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol 2011; 6: 479–507. - PubMed
-
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem 1998; 67: 425–479. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

