Interference of Paraben Compounds with Estrogen Metabolism by Inhibition of 17β-Hydroxysteroid Dehydrogenases
- PMID: 28925944
- PMCID: PMC5618656
- DOI: 10.3390/ijms18092007
Interference of Paraben Compounds with Estrogen Metabolism by Inhibition of 17β-Hydroxysteroid Dehydrogenases
Abstract
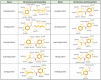
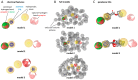
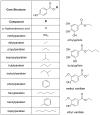
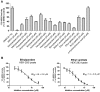
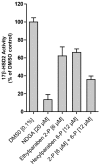
Parabens are effective preservatives widely used in cosmetic products and processed food, with high human exposure. Recent evidence suggests that parabens exert estrogenic effects. This work investigated the potential interference of parabens with the estrogen-activating enzyme 17β-hydroxysteroid dehydrogenase (17β-HSD) 1 and the estrogen-inactivating 17β-HSD2. A ligand-based 17β-HSD2 pharmacophore model was applied to screen a cosmetic chemicals database, followed by in vitro testing of selected paraben compounds for inhibition of 17β-HSD1 and 17β-HSD2 activities. All tested parabens and paraben-like compounds, except their common metabolite p-hydroxybenzoic acid, inhibited 17β-HSD2. Ethylparaben and ethyl vanillate inhibited 17β-HSD2 with IC50 values of 4.6 ± 0.8 and 1.3 ± 0.3 µM, respectively. Additionally, parabens size-dependently inhibited 17β-HSD1, whereby hexyl- and heptylparaben were most active with IC50 values of 2.6 ± 0.6 and 1.8 ± 0.3 µM. Low micromolar concentrations of hexyl- and heptylparaben decreased 17β-HSD1 activity, and ethylparaben and ethyl vanillate decreased 17β-HSD2 activity. However, regarding the very rapid metabolism of these compounds to the inactive p-hydroxybenzoic acid by esterases, it needs to be determined under which conditions low micromolar concentrations of these parabens or their mixtures can occur in target cells to effectively disturb estrogen effects in vivo.
Keywords: 17β-hydroxysteroid dehydrogenase; endocrine disrupting chemical; estrogen; in silico; in vitro; xenobiotic.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data in the writing of the manuscript; and in the decision to publish the results.
Figures



Similar articles
-
Structural determinants of parabens in inhibiting human and rat gonadal 3β-hydroxysteroid dehydrogenase.Toxicol Appl Pharmacol. 2024 Nov;492:117133. doi: 10.1016/j.taap.2024.117133. Epub 2024 Oct 23. Toxicol Appl Pharmacol. 2024. PMID: 39454967
-
Inhibitory effects of parabens on human and rat 17β-hydroxysteroid dehydrogenase 1: Mechanisms of action and impact on hormone synthesis.Toxicology. 2024 Aug;506:153873. doi: 10.1016/j.tox.2024.153873. Epub 2024 Jul 8. Toxicology. 2024. PMID: 38986729
-
Potential antiandrogenic effects of parabens and benzophenone-type UV-filters by inhibition of 3α-hydroxysteroid dehydrogenases.Toxicology. 2024 Dec;509:153997. doi: 10.1016/j.tox.2024.153997. Epub 2024 Nov 10. Toxicology. 2024. PMID: 39532263
-
Inhibitors of 17beta-hydroxysteroid dehydrogenase type 1.Curr Med Chem. 2008;15(2):137-50. doi: 10.2174/092986708783330629. Curr Med Chem. 2008. PMID: 18220769 Review.
-
Inhibitors of 17β-hydroxysteroid dehydrogenase type 1, 2 and 14: Structures, biological activities and future challenges.Mol Cell Endocrinol. 2019 Jun 1;489:66-81. doi: 10.1016/j.mce.2018.10.001. Epub 2018 Oct 15. Mol Cell Endocrinol. 2019. PMID: 30336189 Review.
Cited by
-
17β-Hydroxysteroid Dehydrogenase Type 2 Expression Is Induced by Androgen Signaling in Endometrial Cancer.Int J Mol Sci. 2018 Apr 10;19(4):1139. doi: 10.3390/ijms19041139. Int J Mol Sci. 2018. PMID: 29642629 Free PMC article.
-
Environmental exposure to selected non-persistent endocrine disrupting chemicals and polycystic ovary syndrome: a systematic review.Int J Occup Med Environ Health. 2025 Apr 23;38(2):98-121. doi: 10.13075/ijomeh.1896.02551. Epub 2025 Apr 7. Int J Occup Med Environ Health. 2025. PMID: 40200737 Free PMC article.
-
Natural alternatives from your garden for hair care: Revisiting the benefits of tropical herbs.Heliyon. 2023 Nov 7;9(11):e21876. doi: 10.1016/j.heliyon.2023.e21876. eCollection 2023 Nov. Heliyon. 2023. PMID: 38034771 Free PMC article. Review.
-
Exposure to Propylparaben During Pregnancy and Lactation Induces Long-Term Alterations to the Mammary Gland in Mice.Endocrinology. 2021 Jun 1;162(6):bqab041. doi: 10.1210/endocr/bqab041. Endocrinology. 2021. PMID: 33724348 Free PMC article.
-
The effects of co-exposure to methyl paraben and dibutyl phthalate on cell line derived from human skin.Toxicol Res. 2022 Aug 31;39(1):71-89. doi: 10.1007/s43188-022-00151-3. eCollection 2023 Jan. Toxicol Res. 2022. PMID: 36721678 Free PMC article.
References
-
- Andersen F.A. Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int. J. Toxicol. 2008;27:1–82. - PubMed
-
- Jackson E.M. Moisturizers of today. J. Toxicol. Cutan. Ocul. Toxicol. 1992;11:173–184. doi: 10.3109/15569529209042706. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

