Chimeric antigen receptor T-cell therapy - assessment and management of toxicities
- PMID: 28925994
- PMCID: PMC6733403
- DOI: 10.1038/nrclinonc.2017.148
Chimeric antigen receptor T-cell therapy - assessment and management of toxicities
Abstract
Immunotherapy using T cells genetically engineered to express a chimeric antigen receptor (CAR) is rapidly emerging as a promising new treatment for haematological and non-haematological malignancies. CAR-T-cell therapy can induce rapid and durable clinical responses, but is associated with unique acute toxicities, which can be severe or even fatal. Cytokine-release syndrome (CRS), the most commonly observed toxicity, can range in severity from low-grade constitutional symptoms to a high-grade syndrome associated with life-threatening multiorgan dysfunction; rarely, severe CRS can evolve into fulminant haemophagocytic lymphohistiocytosis (HLH). Neurotoxicity, termed CAR-T-cell-related encephalopathy syndrome (CRES), is the second most-common adverse event, and can occur concurrently with or after CRS. Intensive monitoring and prompt management of toxicities is essential to minimize the morbidity and mortality associated with this potentially curative therapeutic approach; however, algorithms for accurate and consistent grading and management of the toxicities are lacking. To address this unmet need, we formed a CAR-T-cell-therapy-associated TOXicity (CARTOX) Working Group, comprising investigators from multiple institutions and medical disciplines who have experience in treating patients with various CAR-T-cell therapy products. Herein, we describe the multidisciplinary approach adopted at our institutions, and provide recommendations for monitoring, grading, and managing the acute toxicities that can occur in patients treated with CAR-T-cell therapy.
Conflict of interest statement
Competing interests statement
S.S.N. has received research support from Bristol-Myers Squibb, Celgene, Cellectis, Kite Pharma, Merck, and Poseida Therapeutics. S.S.N. has also served as a consultant and/or Scientific Advisory Board member for Celgene, Kite Pharma, Merck, and Novartis. S.T. served as a Scientific Advisory Board member for Kite Pharma. F.L.L. has served as a Scientific Advisory Board member for Kite Pharma, and as a Consultant to Cellular Biomedicine Group. K.V.K. has served as a scientific advisor to and has received research funding from Juno Therapeutics and Kite Pharma. Y.L. has received research funding from Janssen. N.J. has received research support from Abbvie, ADC Therapeutics, Bristol-Myers Squibb, Celgene, Genentech, Incyte, Pharmacyclics, Pfizer, Seattle Genetics, Servier, and Verastem. N.J. has also served on the advisory board and received honorarium from Adaptive Biotechnologies, ADC Therapeutics, Novartis, Novimmune, Pharmacyclics, Pfizer, Servier, and Verastem. N.D. has received research support from Bristol-Myers Squibb, Daichi-Sanky, Incyte, Karyopharm, Pfizer, and Sunesis. N.D. has also received served as a consultant for Incyte, Jazz, Karyopharm, Novartis, Otsuka, Pfizer, and Sunesis. J.W. has received research funding and served on the Advisory Boards for Kite Pharma and Novartis. J.F.d.G. has received research support from Astrazeneca, Deciphera Pharmaceuticals, Eli Lilly, EMD-Serono, Mundipharma, Novartis, Sanofi-Aventis. J.F.d.G. has also served as a consultant or Advisory Board member for AbbVie, Astrazeneca, Celldex, Deciphera Pharmaceuticals, FivePrime Therapeutics, Foundation Medicine, Genentech, Insys Therapeutics, Kadmon, Merck, Novartis, and Novogen. J.F.d.G. is a stock owner of Gilead and Ziopharm Oncology, and his spouse is employed by Ziopharm Oncology. S.A. served as an Advisory Board member for Kite Pharma. K.R. is on the Independent Data Monitoring Committee for Kiadis Pharma. The other authors declare no competing interests.
Figures
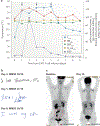
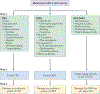
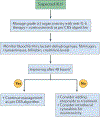
Comment in
-
Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit 'ALL'.Nat Rev Clin Oncol. 2018 Apr;15(4):218. doi: 10.1038/nrclinonc.2018.20. Epub 2018 Feb 13. Nat Rev Clin Oncol. 2018. PMID: 29434334 Free PMC article. No abstract available.
-
Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit 'ALL'.Nat Rev Clin Oncol. 2018 Apr;15(4):218. doi: 10.1038/nrclinonc.2018.19. Epub 2018 Feb 13. Nat Rev Clin Oncol. 2018. PMID: 29434335 No abstract available.
References
-
- June CH, Riddell SR & Schumacher TN Adoptive cellular therapy: a race to the finish line. Sci. Transl Med 7, 280ps7 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

