A critical period for antidepressant-induced acceleration of neuronal maturation in adult dentate gyrus
- PMID: 28925998
- PMCID: PMC5639251
- DOI: 10.1038/tp.2017.208
A critical period for antidepressant-induced acceleration of neuronal maturation in adult dentate gyrus
Abstract
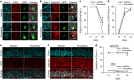
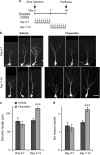
Selective serotonin reuptake inhibitors (SSRIs) are the most commonly used medications for mood and anxiety disorders, and adult neurogenesis in the dentate gyrus has been shown to be involved in the behavioral effects of SSRIs in mice. Studies have shown the varied effects of chronic treatment with SSRIs on adult neurogenesis. One such effect is the acceleration of neuronal maturation, which affects the functional integration of new neurons into existing neuronal circuitry. In this study, we labeled new neurons by using GFP-expressing retroviral vectors in mice and investigated the effect of an SSRI, fluoxetine, on these neurons at different time points after neuronal birth. Chronic treatment with fluoxetine accelerated the dendritic development of the newborn neurons and shifted the timing of the expression of the maturational marker proteins, doublecortin and calbindin. This accelerated maturation was observed even after sub-chronic treatment, only when fluoxetine was administered during the second week of neuronal birth. These results suggest the existence of a 'critical period' for the fluoxetine-induced maturation of new neurons. We propose that the modified functional integration of new neurons in the critical period may underlie the behavioral effects of fluoxetine by regulating anxiety-related decision-making processes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Smith K. Mental health: a world of depression. Nature 2014; 515: 181. - PubMed
-
- Blier P. The pharmacology of putative early-onset antidepressant strategies. Eur Neuropsychopharmacol: J Eur Coll Neuropsychopharmacol 2003; 13: 57–66. - PubMed
-
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003; 301: 805–809. - PubMed
-
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry 2008; 64: 293–301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

