C/EBP homologous protein-induced loss of intestinal epithelial stemness contributes to bile duct ligation-induced cholestatic liver injury in mice
- PMID: 28926118
- PMCID: PMC5859257
- DOI: 10.1002/hep.29540
C/EBP homologous protein-induced loss of intestinal epithelial stemness contributes to bile duct ligation-induced cholestatic liver injury in mice
Abstract
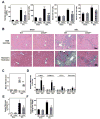
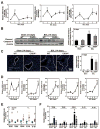
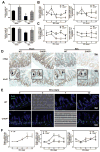
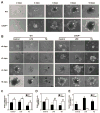
Impaired intestinal barrier function promotes the progression of various liver diseases, including cholestatic liver diseases. The close association of primary sclerosing cholangitis (PSC) with inflammatory bowel disease highlights the importance of the gut-liver axis. It has been reported that bile duct ligation (BDL)-induced liver fibrosis is significantly reduced in C/EBP homologous protein knockout (CHOP-/- ) mice. However, the underlying mechanisms remain unclear. In the current study, we demonstrate that BDL induces striking and acute hepatic endoplasmic reticulum (ER) stress responses after 1 day, which return to normal after 3 days. No significant hepatocyte apoptosis is detected 7-14 days following BDL. However, the inflammatory response is significantly increased after 7 days, which is similar to what we found in human PSC liver samples. BDL-induced loss of stemness in intestinal stem cells (ISCs), disruption of intestinal barrier function, bacterial translocation, activation of hepatic inflammation, M2 macrophage polarization and liver fibrosis are significantly reduced in CHOP-/- mice. In addition, intestinal organoids derived from CHOP-/- mice contain more and longer crypt structures than those from wild-type (WT) mice, which is consistent with the upregulation of stem cell markers (leucine-rich repeat-containing G-protein-coupled receptor 5, olfactomedin 4, and SRY [sex determining region Y]-box 9) and in vivo findings that CHOP-/- mice have longer villi and crypts as compared to WT mice. Similarly, mRNA levels of CD14, interleukin-1β, tumor necrosis factor-alpha, and monocyte chemotactic protein-1 are increased and stem cell proliferation is suppressed in the duodenum of patients with cirrhosis.
Conclusion: Activation of ER stress and subsequent loss of stemness of ISCs plays a critical role in BDL-induced systemic inflammation and cholestatic liver injury. Modulation of the ER stress response represents a potential therapeutic strategy for cholestatic liver diseases as well as other inflammatory diseases. (Hepatology 2018;67:1441-1457).
© 2017 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
None declared.
Figures




Comment in
-
"CHOP"ing intestinal stem cells on way to cholestatic liver injury.Hepatology. 2018 Apr;67(4):1216-1218. doi: 10.1002/hep.29656. Epub 2018 Feb 20. Hepatology. 2018. PMID: 29144555 No abstract available.
Similar articles
-
CHOP deficiency attenuates cholestasis-induced liver fibrosis by reduction of hepatocyte injury.Am J Physiol Gastrointest Liver Physiol. 2008 Feb;294(2):G498-505. doi: 10.1152/ajpgi.00482.2007. Epub 2008 Jan 3. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 18174271
-
Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis.J Biol Chem. 2014 Apr 4;289(14):9584-99. doi: 10.1074/jbc.M113.526517. Epub 2014 Feb 11. J Biol Chem. 2014. PMID: 24519940 Free PMC article.
-
Inflammasome Is Activated in the Liver of Cholestatic Patients and Aggravates Hepatic Injury in Bile Duct-Ligated Mouse.Cell Mol Gastroenterol Hepatol. 2020;9(4):679-688. doi: 10.1016/j.jcmgh.2019.12.008. Epub 2019 Dec 27. Cell Mol Gastroenterol Hepatol. 2020. PMID: 31887435 Free PMC article.
-
Plasma biomarkers of liver injury and inflammation demonstrate a lack of apoptosis during obstructive cholestasis in mice.Toxicol Appl Pharmacol. 2013 Dec 15;273(3):524-31. doi: 10.1016/j.taap.2013.09.023. Epub 2013 Oct 3. Toxicol Appl Pharmacol. 2013. PMID: 24096036 Free PMC article.
-
Mitochondria at the Crossroads of Cholestatic Liver Injury: Targeting Novel Therapeutic Avenues.J Clin Transl Hepatol. 2024 Sep 28;12(9):792-801. doi: 10.14218/JCTH.2024.00087. Epub 2024 Jul 15. J Clin Transl Hepatol. 2024. PMID: 39280065 Free PMC article. Review.
Cited by
-
Activation of cDCs and iNKT cells contributes to triptolide-induced hepatotoxicity via STING signaling pathway and endoplasmic reticulum stress.Cell Biol Toxicol. 2023 Aug;39(4):1753-1772. doi: 10.1007/s10565-022-09782-6. Epub 2022 Dec 15. Cell Biol Toxicol. 2023. PMID: 36520315
-
C/EBP Homologous Protein (CHOP) Activates Macrophages and Promotes Liver Fibrosis in Schistosoma japonicum-Infected Mice.J Immunol Res. 2019 Dec 1;2019:5148575. doi: 10.1155/2019/5148575. eCollection 2019. J Immunol Res. 2019. PMID: 31886304 Free PMC article.
-
The role of sphingosine kinase 2 in alcoholic liver disease.Dig Liver Dis. 2019 Aug;51(8):1154-1163. doi: 10.1016/j.dld.2019.03.020. Epub 2019 Apr 16. Dig Liver Dis. 2019. PMID: 31003959 Free PMC article.
-
Crosstalk Between Bile Acids and Intestinal Epithelium: Multidimensional Roles of Farnesoid X Receptor and Takeda G Protein Receptor 5.Int J Mol Sci. 2025 Apr 29;26(9):4240. doi: 10.3390/ijms26094240. Int J Mol Sci. 2025. PMID: 40362481 Free PMC article. Review.
-
CCAAT/Enhancer-Binding Proteins in Fibrosis: Complex Roles Beyond Conventional Understanding.Research (Wash D C). 2022 Oct 3;2022:9891689. doi: 10.34133/2022/9891689. eCollection 2022. Research (Wash D C). 2022. PMID: 36299447 Free PMC article. Review.
References
-
- Chazouilleres O. Novel Aspects in the Management of Cholestatic Liver Diseases. Dig Dis. 2016;34:340–346. - PubMed
-
- Eksteen B. The Gut-Liver Axis in Primary Sclerosing Cholangitis. Clin Liver Dis. 2016;20:1–14. - PubMed
-
- Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

