MELK is not necessary for the proliferation of basal-like breast cancer cells
- PMID: 28926338
- PMCID: PMC5605198
- DOI: 10.7554/eLife.26693
MELK is not necessary for the proliferation of basal-like breast cancer cells
Abstract
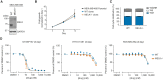
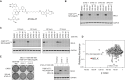
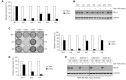
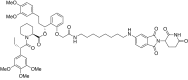
Thorough preclinical target validation is essential for the success of drug discovery efforts. In this study, we combined chemical and genetic perturbants, including the development of a novel selective maternal embryonic leucine zipper kinase (MELK) inhibitor HTH-01-091, CRISPR/Cas9-mediated MELK knockout, a novel chemical-induced protein degradation strategy, RNA interference and CRISPR interference to validate MELK as a therapeutic target in basal-like breast cancers (BBC). In common culture conditions, we found that small molecule inhibition, genetic deletion, or acute depletion of MELK did not significantly affect cellular growth. This discrepancy to previous findings illuminated selectivity issues of the widely used MELK inhibitor OTSSP167, and potential off-target effects of MELK-targeting short hairpins. The different genetic and chemical tools developed here allow for the identification and validation of any causal roles MELK may play in cancer biology, which will be required to guide future MELK drug discovery efforts. Furthermore, our study provides a general framework for preclinical target validation.
Keywords: HTH-01-091; MELK; OTSSP167; basal-like breast cancer; cancer biology; cell biology; human; target validation.
Conflict of interest statement
An inventor on patent PCT Int. Appl. (2016), WO 2016141296 A1 20160909. covering the use of HTH-01-091.
No competing interests declared.
Tinghu Zhang: An inventor on patent PCT Int. Appl. (2016), WO 2016141296 A1 20160909. covering the use of HTH-01-091.
Yubao Wang: An inventor on patent PCT Int. Appl. (2016), WO 2016141296 A1 20160909. covering the use of HTH-01-091.
A scientific founder of Syros Pharmaceuticals, SHAPE Pharmaceuticals, Acetylon Pharmaceuticals, Tensha Therapeutics (now Roche) and C4 Therapeutics and is the inventor on IP licensed to these entities. J.E.B. is now an executive and shareholder in Novartis AG.
Jean J Zhao: An inventor on patent PCT Int. Appl. (2016), WO 2016141296 A1 20160909. covering the use of HTH-01-091.
An inventor on patent PCT Int. Appl. (2016), WO 2016141296 A1 20160909. covering the use of HTH-01-091. A founder of C4 Therapeutics, which has licensed degrader related intellectual property from DFCI.
Figures






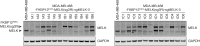



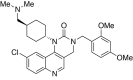


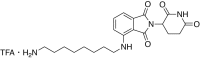

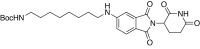
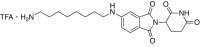
References
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica Section D Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
-
- Beke L, Kig C, Linders JT, Boens S, Boeckx A, van Heerde E, Parade M, De Bondt A, Van den Wyngaert I, Bashir T, Ogata S, Meerpoel L, Van Eynde A, Johnson CN, Beullens M, Brehmer D, Bollen M. MELK-T1, a small-molecule inhibitor of protein kinase MELK, decreases DNA-damage tolerance in proliferating cancer cells. Bioscience Reports. 2015;35:e00267. doi: 10.1042/BSR20150194. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

