Critical developmental windows for morphology and hematology revealed by intermittent and continuous hypoxic incubation in embryos of quail (Coturnix coturnix)
- PMID: 28926567
- PMCID: PMC5604962
- DOI: 10.1371/journal.pone.0183649
Critical developmental windows for morphology and hematology revealed by intermittent and continuous hypoxic incubation in embryos of quail (Coturnix coturnix)
Abstract
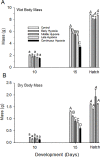
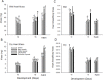
Hypoxia during embryonic growth in embryos is frequently a powerful determinant of development, but at least in avian embryos the effects appear to show considerable intra- and inter-specific variation. We hypothesized that some of this variation may arise from different protocols that may or may not result in exposure during the embryo's critical window for hypoxic effects. To test this hypothesis, quail embryos (Coturnix coturnix) in the intact egg were exposed to hypoxia (~15% O2) during "early" (Day 0 through Day 5, abbreviated as D0-D5), "middle" (D6-D10) or "late" (D11-D15) incubation or for their entire 16-18 day incubation ("continuous hypoxia") to determine critical windows for viability and growth. Viability, body mass, beak and toe length, heart mass, and hematology (hematocrit and hemoglobin concentration) were measured on D5, D10, D15 and at hatching typically between D16 and D18 Viability rate was ~50-70% immediately following the exposure period in the early, middle and late hypoxic groups, but viability improved in the early and late groups once normoxia was restored. Middle hypoxia groups showed continuing low viability, suggesting a critical period from D6-D10 for embryo viability. The continuous hypoxia group experienced viability reaching <10% after D15. Hypoxia, especially during late and continuous hypoxia, also inhibited growth of body, beak and toe when measured at D15. Full recovery to normal body mass upon hatching occurred in all other groups except for continuous hypoxia. Contrary to previous avian studies, heart mass, hematocrit and hemoglobin concentration were not altered by any hypoxic incubation pattern. Although hypoxia can inhibit embryo viability and organ growth during most incubation periods, the greatest effects result from continuous or middle incubation hypoxic exposure. Hypoxic inhibition of growth can subsequently be "repaired" by catch-up growth if a final period of normoxic development is available. Collectively, these data indicate a critical developmental window for hypoxia susceptibility during the mid-embryonic period of development.
Conflict of interest statement
Figures




Similar articles
-
Hypoxic incubation creates differential morphological effects during specific developmental critical windows in the embryo of the chicken (Gallus gallus).Respir Physiol Neurobiol. 2005 Feb 15;145(2-3):251-63. doi: 10.1016/j.resp.2004.09.005. Respir Physiol Neurobiol. 2005. PMID: 15705540
-
Hematology from embryo to adult in the bobwhite quail (Colinus virginianus): Differential effects in the adult of clutch, sex and hypoxic incubation.Comp Biochem Physiol A Mol Integr Physiol. 2018 Apr;218:24-34. doi: 10.1016/j.cbpa.2018.01.005. Epub 2018 Jan 31. Comp Biochem Physiol A Mol Integr Physiol. 2018. PMID: 29369792
-
Chronic hypoxia alters the physiological and morphological trajectories of developing chicken embryos.Comp Biochem Physiol A Mol Integr Physiol. 2002 Apr;131(4):713-24. doi: 10.1016/s1095-6433(02)00009-0. Comp Biochem Physiol A Mol Integr Physiol. 2002. PMID: 11897182
-
Follow Me! A Tale of Avian Heart Development with Comparisons to Mammal Heart Development.J Cardiovasc Dev Dis. 2020 Mar 7;7(1):8. doi: 10.3390/jcdd7010008. J Cardiovasc Dev Dis. 2020. PMID: 32156044 Free PMC article. Review.
-
Effects of Developmental Hypoxia on the Vertebrate Cardiovascular System.Physiology (Bethesda). 2023 Mar 1;38(2):0. doi: 10.1152/physiol.00022.2022. Epub 2022 Nov 1. Physiology (Bethesda). 2023. PMID: 36317939 Review.
Cited by
-
Dietary Exposure to Low Levels of Crude Oil Affects Physiological and Morphological Phenotype in Adults and Their Eggs and Hatchlings of the King Quail (Coturnix chinensis).Front Physiol. 2021 Apr 9;12:661943. doi: 10.3389/fphys.2021.661943. eCollection 2021. Front Physiol. 2021. PMID: 33897469 Free PMC article.
-
Beyond the Chicken: Alternative Avian Models for Developmental Physiological Research.Front Physiol. 2021 Oct 21;12:712633. doi: 10.3389/fphys.2021.712633. eCollection 2021. Front Physiol. 2021. PMID: 34744759 Free PMC article.
-
Phenotypic Switching Resulting From Developmental Plasticity: Fixed or Reversible?Front Physiol. 2020 Jan 22;10:1634. doi: 10.3389/fphys.2019.01634. eCollection 2019. Front Physiol. 2020. PMID: 32038303 Free PMC article. Review.
References
-
- Mueller CA, Burggren WW, Tazawa H (2015) The Physiology of the Avian Embryo In: Whittow GC, editor. Sturkie's Avian Physiology. 6th ed. New York: Elsevier; pp. 739–766.
-
- Altimiras J, Phu L (2000) Lack of physiological plasticity in the early chicken embryo exposed to acute hypoxia. Journal of Experimental Zoology 286: 450–456. - PubMed
-
- Carey C, Thompson EL, Vleck CM, James FC (1982) Avian reproduction over an altitudinal gradient: incubation period, hatchling mass, and embryonic oxygen consumption. Auk 99: 710–718.
-
- Meuer HJ, Hartmann V, Jopp S (1992) Tissue PO2 and growth rate in early chick embryos. Respir Physiol 90: 227–237. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

