Kempopeptin C, a Novel Marine-Derived Serine Protease Inhibitor Targeting Invasive Breast Cancer
- PMID: 28926939
- PMCID: PMC5618429
- DOI: 10.3390/md15090290
Kempopeptin C, a Novel Marine-Derived Serine Protease Inhibitor Targeting Invasive Breast Cancer
Abstract
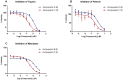
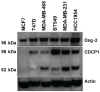
Kempopeptin C, a novel chlorinated analogue of kempopeptin B, was discovered from a marine cyanobacterium collected from Kemp Channel in Florida. The structure was elucidated using NMR spectroscopy and mass spectrometry (MS). The presence of the basic Lys residue adjacent to the N-terminus of the 3-amino-6-hydroxy-2-piperidone (Ahp) moiety contributed to its selectivity towards trypsin and related proteases. The antiproteolytic activity of kempopeptin C was evaluated against trypsin, plasmin and matriptase and found to inhibit these enzymes with IC50 values of 0.19, 0.36 and 0.28 μM, respectively. Due to the significance of these proteases in cancer progression and metastasis, as well as their functional redundancy with respect to targeting overlapping substrates, we examined the effect of kempopeptin C on the downstream cellular substrates of matriptase: CDCP1 and desmoglein-2 (Dsg-2). Kempopeptin C was shown to inhibit the cleavage of both substrates in vitro. Additionally, kempopeptin C reduced the cleavage of CDCP1 in MDA-MB-231 cells up to 10 µM. The functional relevance of targeting matriptase and related proteases was investigated by assessing the effect of kempopeptin C on the migration of breast cancer cells. Kempopeptin C inhibited the migration of the invasive MDA-MB-231 cells by 37 and 60% at 10 and 20 µM, respectively.
Keywords: breast cancer; marine cyanobacteria; migration; serine proteases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Kempopeptins A and B, serine protease inhibitors with different selectivity profiles from a marine cyanobacterium, Lyngbya sp.J Nat Prod. 2008 Sep;71(9):1625-9. doi: 10.1021/np8002172. Epub 2008 Aug 12. J Nat Prod. 2008. PMID: 18693761
-
Hepatocyte growth factor activation inhibitors (HAI-1 and HAI-2) regulate HGF-induced invasion of human breast cancer cells.Int J Cancer. 2006 Sep 1;119(5):1176-83. doi: 10.1002/ijc.21881. Int J Cancer. 2006. PMID: 16557597
-
Molassamide, a depsipeptide serine protease inhibitor from the marine cyanobacterium Dichothrix utahensis.J Nat Prod. 2010 Mar 26;73(3):459-62. doi: 10.1021/np900603f. J Nat Prod. 2010. PMID: 20020755
-
A critical review on marine serine protease and its inhibitors: A new wave of drugs?Int J Biol Macromol. 2021 Feb 15;170:674-687. doi: 10.1016/j.ijbiomac.2020.12.134. Epub 2020 Dec 30. Int J Biol Macromol. 2021. PMID: 33387547 Review.
-
Specifically targeting cancer proliferation and metastasis processes: the development of matriptase inhibitors.Cancer Metastasis Rev. 2019 Sep;38(3):507-524. doi: 10.1007/s10555-019-09802-8. Cancer Metastasis Rev. 2019. PMID: 31471691 Review.
Cited by
-
Understanding the function of the tumor microenvironment, and compounds from marine organisms for breast cancer therapy.World J Biol Chem. 2021 Mar 27;12(2):15-37. doi: 10.4331/wjbc.v12.i2.15. World J Biol Chem. 2021. PMID: 33815682 Free PMC article. Review.
-
Phosphate Limitation Increases Content of Protease Inhibitors in the Cyanobacterium Microcystis aeruginosa.Toxins (Basel). 2020 Jan 6;12(1):33. doi: 10.3390/toxins12010033. Toxins (Basel). 2020. PMID: 31935921 Free PMC article.
-
Natural Compounds as Protease Inhibitors in Therapeutic Focus on Cancer Therapy.Anticancer Agents Med Chem. 2024;24(16):1167-1181. doi: 10.2174/0118715206303964240708095110. Anticancer Agents Med Chem. 2024. PMID: 38988167 Review.
-
Marine Organisms as a Prolific Source of Bioactive Depsipeptides.Mar Drugs. 2023 Feb 11;21(2):120. doi: 10.3390/md21020120. Mar Drugs. 2023. PMID: 36827161 Free PMC article. Review.
-
Discovery, Synthesis, Pharmacological Profiling, and Biological Characterization of Brintonamides A-E, Novel Dual Protease and GPCR Modulators from a Marine Cyanobacterium.J Med Chem. 2018 Jul 26;61(14):6364-6378. doi: 10.1021/acs.jmedchem.8b00885. Epub 2018 Jul 17. J Med Chem. 2018. PMID: 30015488 Free PMC article.
References
-
- Netzel-arnett S., Hooper J.D., Szabo R., Madison E.L., Quigley J.P., Bugge H., Antalis T.M. Membrane anchored serine proteases: A rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metastasis Rev. 2003;22:237–258. doi: 10.1023/A:1023003616848. - DOI - PubMed
-
- Shi Y.E., Torri J., Yieh L., Wellstein A., Lippman M.E., Dickson R.B. Identification and characterization of a novel matrix-degrading protease from hormone-dependent human breast cancer cells. Cancer Res. 1993;53:1409–1415. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

