Emerging Roles of Heparanase in Viral Pathogenesis
- PMID: 28927006
- PMCID: PMC5618000
- DOI: 10.3390/pathogens6030043
Emerging Roles of Heparanase in Viral Pathogenesis
Abstract
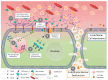
Heparan sulfate (HS) is ubiquitously expressed on mammalian cells. It is a polysaccharide that binds growth factors, cytokines, and chemokines, and thereby controls several important physiological functions. Ironically, many human pathogens including viruses interact with it for adherence to host cells. HS functions can be regulated by selective modifications and/or selective cleavage of the sugar chains from the cell surface. In mammals, heparanase (HPSE) is the only known enzyme capable of regulating HS functions via a selective endoglycosidase activity that cleaves polymeric HS chains at internal sites. During homeostasis, HPSE expression and its endoglycosidase activity are tightly regulated; however, under stress conditions, including infection, its expression may be upregulated, which could contribute directly to the onset of several disease pathologies. Here we focus on viral infections exemplified by herpes simplex virus, dengue virus, human papillomavirus, respiratory syncytial virus, adenovirus, hepatitis C virus, and porcine respiratory and reproductive syncytial virus to summarize recent advances in understanding the highly significant, but emerging roles, of the enzyme HPSE in viral infection, spread and pathogenesis.
Keywords: heparan sulfate; heparanase; herpes simplex virus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

