Oxidative toxicity in diabetes and Alzheimer's disease: mechanisms behind ROS/ RNS generation
- PMID: 28927401
- PMCID: PMC5606025
- DOI: 10.1186/s12929-017-0379-z
Oxidative toxicity in diabetes and Alzheimer's disease: mechanisms behind ROS/ RNS generation
Abstract
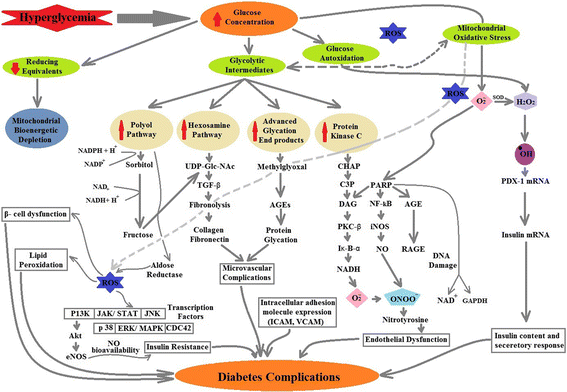
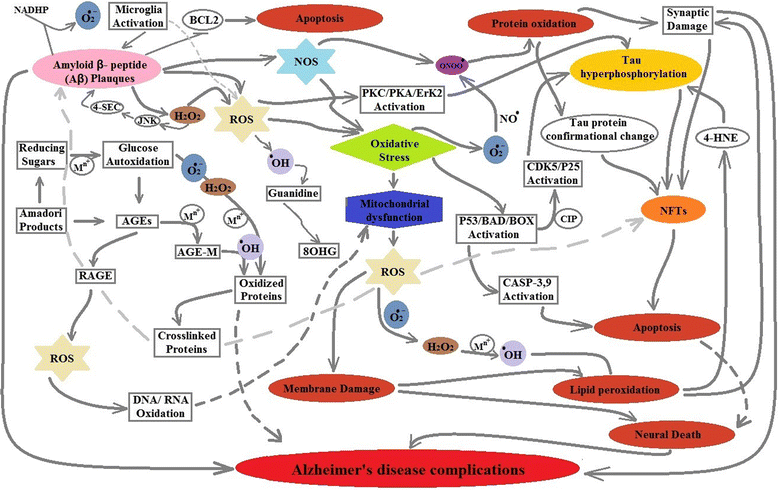
Reactive oxidative species (ROS) toxicity remains an undisputed cause and link between Alzheimer's disease (AD) and Type-2 Diabetes Mellitus (T2DM). Patients with both AD and T2DM have damaged, oxidized DNA, RNA, protein and lipid products that can be used as possible disease progression markers. Although the oxidative stress has been anticipated as a main cause in promoting both AD and T2DM, multiple pathways could be involved in ROS production. The focus of this review is to summarize the mechanisms involved in ROS production and their possible association with AD and T2DM pathogenesis and progression. We have also highlighted the role of current treatments that can be linked with reduced oxidative stress and damage in AD and T2DM.
Keywords: Alzheimer’s disease; Anti-diabetic drugs; Antioxidant treatments; Oxidative stress; ROS production; Type-2 diabetes mellitus.
Conflict of interest statement
Authors information
Waqar Ahmad is working as Research Officer while Khadija Shabbiri is post-doctoral Research fellow at School of Biological Sciences, University of Queensland, Australia. Bushra Ijaz is Assistant professor at CEMB, University of the Punjab, Lahore, Sidra Rehman is Assistant professor at COMSATS; while Fayyaz Ahmed is PhD a student.
Ethics approval and consent to participate
Not Applicable.
Consent for publication
The authors declare that this article is original and never been published before and not submitted to any other journal.
Competing interests
The authors have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Hallmarks of protein oxidative damage in neurodegenerative diseases: focus on Alzheimer's disease.Amino Acids. 2007;32(4):553-9. doi: 10.1007/s00726-006-0431-x. Epub 2007 Feb 2. Amino Acids. 2007. PMID: 17273806 Review.
-
Reactive oxygen and nitrogen species in Alzheimer's disease.Curr Alzheimer Res. 2004 May;1(2):111-9. doi: 10.2174/1567205043332162. Curr Alzheimer Res. 2004. PMID: 15975075 Review.
-
Phytochemical Ginkgolide B Attenuates Amyloid-β1-42 Induced Oxidative Damage and Altered Cellular Responses in Human Neuroblastoma SH-SY5Y Cells.J Alzheimers Dis. 2017;60(s1):S25-S40. doi: 10.3233/JAD-161086. J Alzheimers Dis. 2017. PMID: 28234255
-
Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich's ataxia.J Neurol Sci. 2005 Jun 15;233(1-2):145-62. doi: 10.1016/j.jns.2005.03.012. J Neurol Sci. 2005. PMID: 15896810 Review.
-
In vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as Alzheimer's disease, Parkinson's disease, and diabetes.Free Radic Res. 2017 Apr;51(4):413-427. doi: 10.1080/10715762.2017.1315114. Epub 2017 Apr 25. Free Radic Res. 2017. PMID: 28372523 Review.
Cited by
-
Multi-Target Mechanisms of Phytochemicals in Alzheimer's Disease: Effects on Oxidative Stress, Neuroinflammation and Protein Aggregation.J Pers Med. 2022 Sep 15;12(9):1515. doi: 10.3390/jpm12091515. J Pers Med. 2022. PMID: 36143299 Free PMC article. Review.
-
Involvement of glutathione peroxidases in the occurrence and development of breast cancers.J Transl Med. 2020 Jun 22;18(1):247. doi: 10.1186/s12967-020-02420-x. J Transl Med. 2020. PMID: 32571353 Free PMC article. Review.
-
A Curcumin Analog Exhibits Multiple Biologic Effects on the Pathogenesis of Alzheimer's Disease and Improves Behavior, Inflammation, and β-Amyloid Accumulation in a Mouse Model.Int J Mol Sci. 2020 Jul 30;21(15):5459. doi: 10.3390/ijms21155459. Int J Mol Sci. 2020. PMID: 32751716 Free PMC article.
-
The effect of acupuncture on oxidative stress: A systematic review and meta-analysis of animal models.PLoS One. 2022 Sep 9;17(9):e0271098. doi: 10.1371/journal.pone.0271098. eCollection 2022. PLoS One. 2022. PMID: 36084019 Free PMC article.
-
Improved synthesis of an ergothioneine PET radioligand for imaging oxidative stress in Alzheimer's disease.FEBS Lett. 2022 May;596(10):1279-1289. doi: 10.1002/1873-3468.14303. Epub 2022 Feb 7. FEBS Lett. 2022. PMID: 35100442 Free PMC article.
References
-
- Mulukutla BC, et al. Regulation of Glucose Metabolism - A Perspective From Cell Bioprocessing. Trends Biotechnol. 2016;34(8):638-51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

