Generation of special autosomal dominant polycystic kidney disease iPSCs with the capability of functional kidney-like cell differentiation
- PMID: 28927462
- PMCID: PMC5606115
- DOI: 10.1186/s13287-017-0645-8
Generation of special autosomal dominant polycystic kidney disease iPSCs with the capability of functional kidney-like cell differentiation
Abstract
Background: Human induced pluripotent stem cells (iPSCs) have been verified as a powerful cell model for the study of pathogenesis in hereditary disease. Autosomal dominant polycystic kidney disease (ADPKD) is caused by mutations of PKD or non-PKD genes. The pathogenesis of ADPKD remains unexplored because of the lack of a true human cell model.
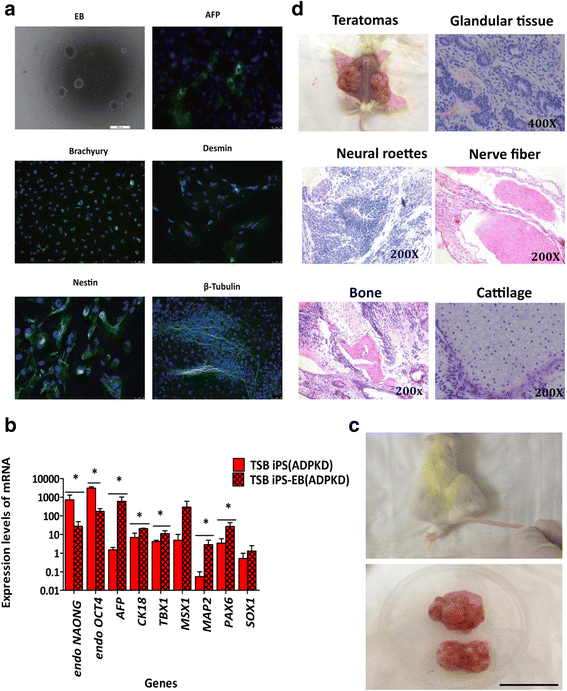
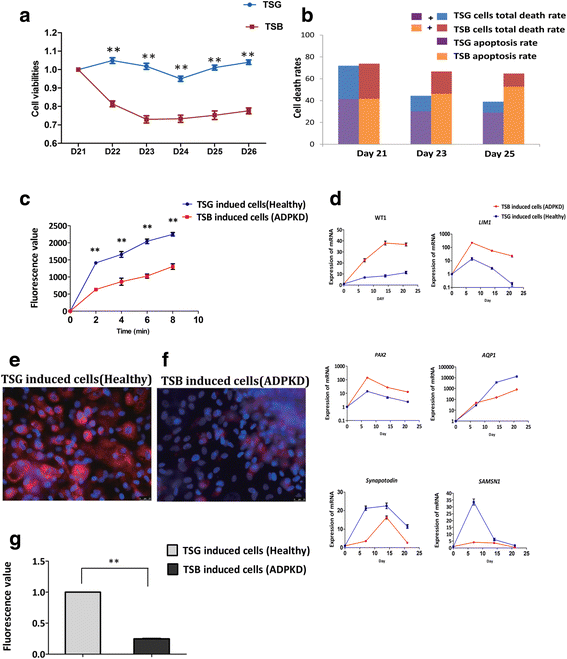
Methods: Six ADPKD patients and four healthy individuals were recruited as donors of somatic cells from a Chinese ADPKD family without mutations of the PKD genes but carrying SAMSN1 gene deletion. The ADPKD-iPSCs were generated from somatic cells and were induced into kidney-like cells (KLCs) by a novel three-step method involving cytokines and renal epithelium growth medium. Furthermore, we analyzed functional properties of these KLCs by water transportation and albumin absorption assays.
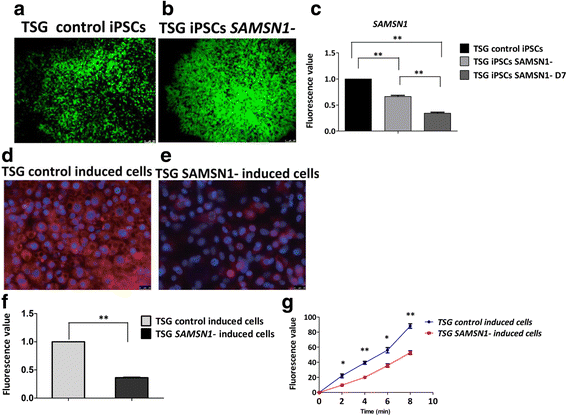
Results: We successfully generated iPSCs from ADPKD patients and differentiated them into KLCs that showed morphological and functional characteristics of human kidney cells. Further, we also found that ADPKD-iPSC-KLCs had a significantly higher rate of apoptosis and a significantly lower capacity for water transportation and albumin absorption compared to healthy sibling-derived differentiated KLCs. Furthermore, knockdown of SAMSN1 in control iPSCs may attenuate differentiation and/or function of KLCs.
Conclusions: These data show that we have created the first iPSCs established from ADPKD patients without mutations in the PKD genes, and suggest that the deletion mutation of SAMSN1 might be involved in the differentiation and/or function of KLCs. ADPKD-iPSC-KLCs can be used as a versatile model system for the study of kidney disease.
Keywords: Autosomal-dominant polycystic kidney disease; Differentiation; Induced pluripotent stem cells; Kidney cells; SAMSN1.
Conflict of interest statement
Ethics approval and consent to participate
All procedures of experiments were approved by the Ethics Committee of Nanchang University Affiliated first Hospital (Additional file 4: Ethical approval). Written informed consent was obtained from all donors.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Cystic renal-epithelial derived induced pluripotent stem cells from polycystic kidney disease patients.Stem Cells Transl Med. 2020 Apr;9(4):478-490. doi: 10.1002/sctm.18-0283. Epub 2020 Mar 12. Stem Cells Transl Med. 2020. PMID: 32163234 Free PMC article.
-
Reduced ciliary polycystin-2 in induced pluripotent stem cells from polycystic kidney disease patients with PKD1 mutations.J Am Soc Nephrol. 2013 Oct;24(10):1571-86. doi: 10.1681/ASN.2012111089. Epub 2013 Sep 5. J Am Soc Nephrol. 2013. PMID: 24009235 Free PMC article.
-
Induced pluripotent stem cells derived from an autosomal dominant polycystic kidney disease patient carrying a PKD1 Q533X mutation.Stem Cell Res. 2017 Dec;25:83-87. doi: 10.1016/j.scr.2017.10.026. Epub 2017 Oct 28. Stem Cell Res. 2017. PMID: 29121521
-
Regulation of ciliary trafficking of polycystin-2 and the pathogenesis of autosomal dominant polycystic kidney disease.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010 Feb;35(2):93-9. doi: 10.3969/j.issn.1672-7347.2010.02.001. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010. PMID: 20197605 Review.
-
Cilia and polycystic kidney disease.Semin Cell Dev Biol. 2021 Feb;110:139-148. doi: 10.1016/j.semcdb.2020.05.003. Epub 2020 May 28. Semin Cell Dev Biol. 2021. PMID: 32475690 Review.
Cited by
-
Lama2 And Samsn1 Mediate the Effects of Brn4 on Hippocampal Neural Stem Cell Proliferation and Differentiation.Stem Cells Int. 2023 Apr 13;2023:7284986. doi: 10.1155/2023/7284986. eCollection 2023. Stem Cells Int. 2023. PMID: 37091532 Free PMC article.
-
Fundamental insights into autosomal dominant polycystic kidney disease from human-based cell models.Pediatr Nephrol. 2019 Oct;34(10):1697-1715. doi: 10.1007/s00467-018-4057-5. Epub 2018 Sep 13. Pediatr Nephrol. 2019. PMID: 30215095
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

