Biochemistry of Mitochondrial Coenzyme Q Biosynthesis
- PMID: 28927698
- PMCID: PMC5731490
- DOI: 10.1016/j.tibs.2017.06.008
Biochemistry of Mitochondrial Coenzyme Q Biosynthesis
Abstract
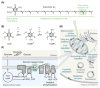
Coenzyme Q (CoQ, ubiquinone) is a redox-active lipid produced across all domains of life that functions in electron transport and oxidative phosphorylation and whose deficiency causes human diseases. Yet, CoQ biosynthesis has not been fully defined in any organism. Several proteins with unclear molecular functions facilitate CoQ biosynthesis through unknown means, and multiple steps in the pathway are catalyzed by currently unidentified enzymes. Here we highlight recent progress toward filling these knowledge gaps through both traditional biochemistry and cutting-edge 'omics' approaches. To help fill the remaining gaps, we present questions framed by the recently discovered CoQ biosynthetic complex and by putative biophysical barriers. Mapping CoQ biosynthesis, metabolism, and transport pathways has great potential to enhance treatment of numerous human diseases.
Keywords: CoQ-synthome; biosynthesis; coenzyme Q; complex Q; lipids; metabolon; mitochondria; mitochondrial disease; oxidative phosphorylation; protein complex; ubiquinone.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures





References
-
- Crane FL, et al. Isolation of a quinone from beef heart mitochondria. Biochim Biophys Acta. 1957;25:220–221. - PubMed
-
- Morton RA. Ubiquinone. Nature. 1958;182:1764–1767. - PubMed
-
- Lester RL, et al. Coenzyme Q: a new group of quinones. Journal of the American Chemical Society. 1958;80:4751–4752.
-
- Wolf DEHCH, Trenner NR, Arison BH, Shunk CH, Linn BO, McPherson JF, Folkers K. Coenzyme Q. I. Structure Studies on the Coenzyme Q Group. J Am Chem Soc. 1958;80:4752.
-
- Mitchell P. Protonmotive redox mechanism of the cytochrome b-c1 complex in the respiratory chain: protonmotive ubiquinone cycle. FEBS Lett. 1975;56:1–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

