Sex-dependent behavioral impairments in the HdhQ350/+ mouse line
- PMID: 28927719
- PMCID: PMC5659761
- DOI: 10.1016/j.bbr.2017.09.026
Sex-dependent behavioral impairments in the HdhQ350/+ mouse line
Abstract
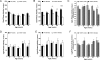
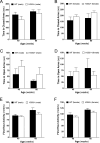
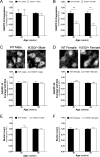
Huntington's Disease (HD) is an autosomal dominant neurodegenerative disease characterized by gradual deterioration of motor and cognitive functions and development of psychiatric deficits. Animal models provide powerful means to study the pathological processes, molecular dysfunctions and symptoms associated with HD. We performed a longitudinal behavioral study of the newly developed HdhQ350/+ mouse line, a knock-in model that expresses a repeat of 350 glutamines. We found remarkable sex-dependent differences on symptom onset and severity. While both sexes lose weight and grip strength, only HdhQ350/+ males have impaired motor coordination as measured by the rotarod and alterations in gait as measured by the catwalk assay. While HdhQ350/+ females do not exhibit impairment in motor coordination, we found a reduction in dark phase locomotor activity. Male and female HdhQ350/+ mice do not show anxiety as measured by the elevated plus maze or changes in exploration as measured by the open field test. To investigate these sex-dependent differences, we performed western blot analyses of striatal tissue. We measured equal mutant huntingtin protein expression in both sexes and found evidence of aggregation. We found the expected decrease of DARPP-32 expression only in female HdhQ350/+ mice. Remarkably, we found no evidence of reduction in synaptophysin or CB1 receptors in HdhQ350/+ tissue of either sex. Our study indicates that male and female HdhQ350/+ mice differentially recapitulate select behavioral impairments commonly measured in other HD mouse models with limited sex-dependent changes in recognized histopathological markers. We conclude that expanded polyglutamine repeats influence HD pathogenesis in a sex-dependent manner.
Keywords: Huntington’s disease; Mouse model; Neurodegeneration; Polyglutamine disease; Protein aggregation; Sex-dependent.
Published by Elsevier B.V.
Conflict of interest statement
All authors report no conflict of interest.
Figures





References
-
- MacDonald ME, et al. Cell. Elsevier; 1993. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes; pp. 971–983. - PubMed
-
- Walker FO. Lancet. Elsevier; 2007. Huntington's disease; pp. 218–228. - PubMed
-
- Ross CA, Tabrizi SJ. The Lancet Neurology. Elsevier; 2011. Huntington's disease: from molecular pathogenesis to clinical treatment; pp. 83–98. - PubMed
-
- Mangiarini L, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996:493–506. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

