A novel IKAROS haploinsufficiency kindred with unexpectedly late and variable B-cell maturation defects
- PMID: 28927821
- PMCID: PMC6588539
- DOI: 10.1016/j.jaci.2017.08.019
A novel IKAROS haploinsufficiency kindred with unexpectedly late and variable B-cell maturation defects
Conflict of interest statement
Disclosure of potential conflict of interest: D. J. Bogaert received a PhD fellowship grant from Research Foundation Flanders (FWO) for this work. U. Cytlak’s and V. Bigley’s institutions received grant 101155/Z/13/Z from the Wellcome Trust for this work. E. De Baere personally received grants from Research Foundation Flanders (FWO, Senior Clinical Investigator), grant number BOF15/GOA/011 from the Ghent University Special Research Fund, and grant number AUGE/13/023 from the Hercules Foundation for this work. F. Haerynck personally received a grant from the Jeffrey Modell Foundation (JMF) for this work. The rest of the authors declare that they have no relevant conflicts of interest.
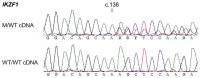
Figures





References
-
- John LB, Ward AC. The Ikaros gene family: transcriptional regulators of hematopoiesis and immunity. Mol Immunol. 2011;48:1272–8. - PubMed
-
- Hoshino A, Okada S, Yoshida K, Nishida N, Okuno Y, Ueno H, et al. Abnormal hematopoiesis and autoimmunity in human subjects with germline IKZF1 mutations. J Allergy Clin Immunol. 2017;140:223–31. - PubMed
-
- Kakinuma S, Kodama Y, Amasaki Y, Yi S, Tokairin Y, Arai M, et al. Ikaros is a mutational target for lymphomagenesis in Mlh1-deficient mice. Oncogene. 2007;26:2945–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

