Chimeric antigen receptor T-cell therapies for multiple myeloma
- PMID: 28928126
- PMCID: PMC5731088
- DOI: 10.1182/blood-2017-06-793869
Chimeric antigen receptor T-cell therapies for multiple myeloma
Abstract
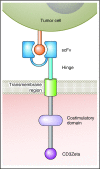
Multiple myeloma (MM) is a nearly always incurable malignancy of plasma cells, so new approaches to treatment are needed. T-cell therapies are a promising approach for treating MM, with a mechanism of action different than those of standard MM treatments. Chimeric antigen receptors (CARs) are fusion proteins incorporating antigen-recognition domains and T-cell signaling domains. T cells genetically engineered to express CARs can specifically recognize antigens. Success of CAR-T cells (CAR-Ts) against leukemia and lymphoma has encouraged development of CAR-T therapies for MM. Target antigens for CARs must be expressed on malignant cells, but expression on normal cells must be absent or limited. B-cell maturation antigen is expressed by normal and malignant plasma cells. CAR-Ts targeting B-cell maturation antigen have demonstrated significant antimyeloma activity in early clinical trials. Toxicities in these trials, including cytokine release syndrome, have been similar to toxicities observed in CAR-T trials for leukemia. Targeting postulated CD19+ myeloma stem cells with anti-CD19 CAR-Ts is a novel approach to MM therapy. MM antigens including CD138, CD38, signaling lymphocyte-activating molecule 7, and κ light chain are under investigation as CAR targets. MM is genetically and phenotypically heterogeneous, so targeting of >1 antigen might often be required for effective treatment of MM with CAR-Ts. Integration of CAR-Ts with other myeloma therapies is an important area of future research. CAR-T therapies for MM are at an early stage of development but have great promise to improve MM treatment.
Conflict of interest statement
Conflict-of-interest disclosure: J.N.K. receives research funding from cooperative research and development agreements between the National Cancer Institute (NCI) and Kite Pharma Inc, and between the NCI and Bluebird Bio Inc, and also has multiple patent applications related to CARs. L.M. declares no competing financial interests.
Figures

References
-
- American Cancer Society. Cancer facts and figures. Atlanta, GA: American Cancer Society; 2017.
-
- Moreau P, Attal M, Facon T. Frontline therapy of multiple myeloma. Blood. 2015;125(20):3076-3084. - PubMed
-
- Laubach J, Garderet L, Mahindra A, et al. . Management of relapsed multiple myeloma: recommendations of the International Myeloma Working Group. Leukemia. 2016;30(5):1005-1017. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

