Endothelial Epas1 Deficiency Is Sufficient To Promote Parietal Epithelial Cell Activation and FSGS in Experimental Hypertension
- PMID: 28928136
- PMCID: PMC5698059
- DOI: 10.1681/ASN.2016090960
Endothelial Epas1 Deficiency Is Sufficient To Promote Parietal Epithelial Cell Activation and FSGS in Experimental Hypertension
Abstract
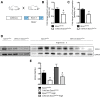
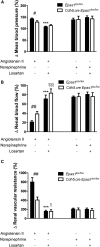
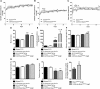
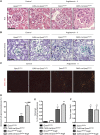
FSGS, the most common primary glomerular disorder causing ESRD, is a complex disease that is only partially understood. Progressive sclerosis is a hallmark of FSGS, and genetic tracing studies have shown that parietal epithelial cells participate in the formation of sclerotic lesions. The loss of podocytes triggers a focal activation of parietal epithelial cells, which subsequently form cellular adhesions with the capillary tuft. However, in the absence of intrinsic podocyte alterations, the origin of the pathogenic signal that triggers parietal epithelial cell recruitment remains elusive. In this study, investigation of the role of the endothelial PAS domain-containing protein 1 (EPAS1), a regulatory α subunit of the hypoxia-inducible factor complex, during angiotensin II-induced hypertensive nephropathy provided novel insights into FSGS pathogenesis in the absence of a primary podocyte abnormality. We infused angiotensin II into endothelial-selective Epas1 knockout mice and their littermate controls. Although the groups presented with identical high BP, endothelial-specific Epas1 gene deletion accentuated albuminuria with severe podocyte lesions and recruitment of pathogenic parietal glomerular epithelial cells. These lesions and dysfunction of the glomerular filtration barrier were associated with FSGS in endothelial Epas1-deficient mice only. These results indicate that endothelial EPAS1 has a global protective role during glomerular hypertensive injuries without influencing the hypertensive effect of angiotensin II. Furthermore, these findings provide proof of principle that endothelial-derived signaling can trigger FSGS and illustrate the potential importance of the EPAS1 endothelial transcription factor in secondary FSGS.
Keywords: Pathophysiology of Renal Disease and Progression; endothelial cells; focal segmental glomerulosclerosis; glomerular endothelial cells; glomerular epithelial cells; hypertension.
Copyright © 2017 by the American Society of Nephrology.
Figures

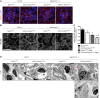
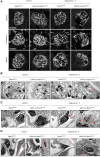
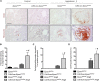
Comment in
-
Glomerular disease: Loss of Epas1 promotes FSGS.Nat Rev Nephrol. 2017 Nov;13(11):663. doi: 10.1038/nrneph.2017.143. Epub 2017 Oct 9. Nat Rev Nephrol. 2017. PMID: 28989175 No abstract available.
References
-
- Strupler M, Hernest M, Fligny C, Martin JL, Tharaux PL, Schanne-Klein MC: Second harmonic microscopy to quantify renal interstitial fibrosis and arterial remodeling. J Biomed Opt 13: 054041, 2008 - PubMed
-
- Crowley SD, Song YS, Sprung G, Griffiths R, Sparks M, Yan M, Burchette JL, Howell DN, Lin EE, Okeiyi B, Stegbauer J, Yang Y, Tharaux PL, Ruiz P: A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension 55: 99–108, 2010 - PMC - PubMed
-
- Nijenhuis T, Sloan AJ, Hoenderop JG, Flesche J, van Goor H, Kistler AD, Bakker M, Bindels RJ, de Boer RA, Möller CC, Hamming I, Navis G, Wetzels JF, Berden JH, Reiser J, Faul C, van der Vlag J: Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am J Pathol 179: 1719–1732, 2011 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

