REACH: study protocol of a randomised trial of rehabilitation very early in congenital hemiplegia
- PMID: 28928195
- PMCID: PMC5623522
- DOI: 10.1136/bmjopen-2017-017204
REACH: study protocol of a randomised trial of rehabilitation very early in congenital hemiplegia
Abstract
Objectives: Congenital hemiplegia is the most common form of cerebral palsy (CP). Children with unilateral CP show signs of upper limb asymmetry by 8 months corrected age (ca) but are frequently not referred to therapy until after 12 months ca. This study compares the efficacy of infant-friendly modified constraint-induced movement therapy (Baby mCIMT) to infant friendly bimanual therapy (Baby BIM) on upper limb, cognitive and neuroplasticity outcomes in a multisite randomised comparison trial.
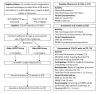
Methods and analysis: 150 infants (75 in each group), aged between 3 and 6 months ca, with asymmetric brain injury and clinical signs of upper extremity asymmetry will be recruited. Children will be randomised centrally to receive equal doses of either Baby mCIMT or Baby BIM. Baby mCIMT comprises restraint of the unimpaired hand using a simple restraint (eg, glove, sock), combined with intensive parent implemented practice focusing on active use of the impaired hand in a play-based context. In contrast, Baby BIM promotes active play requiring both hands in a play-based context. Both interventions will be delivered by parents at home with monthly home visits and interim telecommunication support by study therapists. Assessments will be conducted at study entry; at 6, 12 months ca immediately postintervention (primary outcome) and 24 months ca (retention). The primary outcome will be the Mini-Assisting Hand Assessment. Secondary outcomes include the Bayley Scale for Infant and Toddler Development (cognitive and motor domains) and the Hand Assessment of Infants. A subset of children will undertake MRI scans at 24 months ca to evaluate brain lesion severity and brain (re)organisation after intervention.
Ethics and dissemination: Full ethical approvals for this study have been obtained from the relevant sites. The findings will be disseminated in peer-reviewed publications.
Trial registration number: Australian and New Zealand Clinical Trials Registry: ACTRN12615000180516, Pre results.
Keywords: bimanual therapy; brain injury; cerebral palsy; congenital hemiplegia; constraint induced movement therapy; early intervention; infant.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Stanley F, Blair E. Cerebral palsies: epidemiology and causal pathways: MacKeith Press, 200.
-
- Access Economics. The economic impact of cerebral palsy in Australia in 2007. Canberra 2008.
-
- Sakzewski L, Ziviani J, Boyd R. Efficacy of upper limb interventions for children with unilateral cerebral palsy: systematic review and meta-analysis update. Pediatrics 2014;133:e175–204. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
