Microbial Metabolic Networks at the Mucus Layer Lead to Diet-Independent Butyrate and Vitamin B12 Production by Intestinal Symbionts
- PMID: 28928206
- PMCID: PMC5605934
- DOI: 10.1128/mBio.00770-17
Microbial Metabolic Networks at the Mucus Layer Lead to Diet-Independent Butyrate and Vitamin B12 Production by Intestinal Symbionts
Abstract
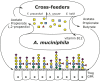
Akkermansia muciniphila has evolved to specialize in the degradation and utilization of host mucus, which it may use as the sole source of carbon and nitrogen. Mucus degradation and fermentation by A. muciniphila are known to result in the liberation of oligosaccharides and subsequent production of acetate, which becomes directly available to microorganisms in the vicinity of the intestinal mucosa. Coculturing experiments of Amuciniphila with non-mucus-degrading butyrate-producing bacteria Anaerostipes caccae, Eubacterium hallii, and Faecalibacterium prausnitzii resulted in syntrophic growth and production of butyrate. In addition, we demonstrate that the production of pseudovitamin B12 by E. hallii results in production of propionate by A. muciniphila, which suggests that this syntrophy is indeed bidirectional. These data are proof of concept for syntrophic and other symbiotic microbe-microbe interactions at the intestinal mucosal interface. The observed metabolic interactions between Amuciniphila and butyrogenic bacterial taxa support the existence of colonic vitamin and butyrate production pathways that are dependent on host glycan production and independent of dietary carbohydrates. We infer that the intestinal symbiont A. muciniphila can indirectly stimulate intestinal butyrate levels in the vicinity of the intestinal epithelial cells with potential health benefits to the host.IMPORTANCE The intestinal microbiota is said to be a stable ecosystem where many networks between microorganisms are formed. Here we present a proof of principle study of microbial interaction at the intestinal mucus layer. We show that indigestible oligosaccharide chains within mucus become available for a broad range of intestinal microbes after degradation and liberation of sugars by the species Akkermansia muciniphila This leads to the microbial synthesis of vitamin B12, 1,2-propanediol, propionate, and butyrate, which are beneficial to the microbial ecosystem and host epithelial cells.
Keywords: Akkermansia muciniphila; anaerobes; butyrate; cross-feeding; intestine; microbiome; mucus; syntrophy.
Copyright © 2017 Belzer et al.
Figures



References
-
- Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, Lieber A, Wu F, Perez-Perez GI, Chen Y, Schweizer W, Zheng X, Contreras M, Dominguez-Bello MG, Blaser MJ. 2016. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med 8:343ra382. doi:10.1126/scitranslmed.aad7121. - DOI - PMC - PubMed
-
- O’Keefe SJ, Li JV, Lahti L, Ou J, Carbonero F, Mohammed K, Posma JM, Kinross J, Wahl E, Ruder E, Vipperla K, Naidoo V, Mtshali L, Tims S, Puylaert PG, DeLany J, Krasinskas A, Benefiel AC, Kaseb HO, Newton K, Nicholson JK, de Vos WM, Gaskins HR, Zoetendal EG. 2015. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun 6:6342. doi:10.1038/ncomms7342. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
