Picornaviruses and Apoptosis: Subversion of Cell Death
- PMID: 28928208
- PMCID: PMC5605936
- DOI: 10.1128/mBio.01009-17
Picornaviruses and Apoptosis: Subversion of Cell Death
Abstract
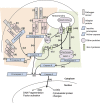
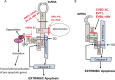
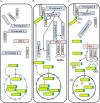
Infected cells can undergo apoptosis as a protective response to viral infection, thereby limiting viral infection. As viruses require a viable cell for replication, the death of the cell limits cellular functions that are required for virus replication and propagation. Picornaviruses are single-stranded RNA viruses that modify the host cell apoptotic response, probably in order to promote viral replication, largely as a function of the viral proteases 2A, 3C, and 3CD. These proteases are essential for viral polyprotein processing and also cleave cellular proteins. Picornavirus proteases cleave proapoptotic adaptor proteins, resulting in downregulation of apoptosis. Picornavirus proteases also cleave nucleoporins, disrupting the orchestrated manner in which signaling pathways use active nucleocytoplasmic trafficking, including those involved in apoptosis. In addition to viral proteases, the transmembrane 2B protein alters intracellular ion signaling, which may also modulate apoptosis. Overall, picornaviruses, via the action of virally encoded proteins, exercise intricate control over and subvert cell death pathways, specifically apoptosis, thereby allowing viral replication to continue.
Keywords: apoptosis; innate immunity; picornavirus; proteases; virus-host interactions.
Copyright © 2017 Croft et al.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
