Carbonic anhydrase III protects osteocytes from oxidative stress
- PMID: 28928248
- PMCID: PMC6266636
- DOI: 10.1096/fj.201700485RR
Carbonic anhydrase III protects osteocytes from oxidative stress
Abstract
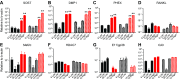
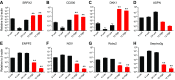
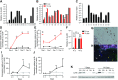
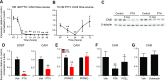
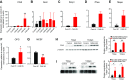
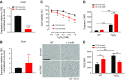
Osteocytes are master orchestrators of bone remodeling; they control osteoblast and osteoclast activities both directly via cell-to-cell communication and indirectly via secreted factors, and they are the main postnatal source of sclerostin and RANKL (receptor activator of NF-kB ligand), two regulators of osteoblast and osteoclast function. Despite progress in understanding osteocyte biology and function, much remains to be elucidated. Recently developed osteocytic cell lines-together with new genome editing tools-has allowed a closer look at the biology and molecular makeup of these cells. By using single-cell cloning, we identified genes that are associated with high Sost/sclerostin expression and analyzed their regulation and function. Unbiased transcriptome analysis of high- vs. low-Sost/sclerostin-expressing cells identified known and novel genes. Dmp1 (dentin matrix protein 1), Dkk1 (Dickkopf WNT signaling pathway inhibitor 1), and Phex were among the most up-regulated known genes, whereas Srpx2, Cd200, and carbonic anhydrase III (CAIII) were identified as novel markers of differentiated osteocytes. Aspn, Enpp2, Robo2, Nov, and Serpina3g were among the transcripts that were most significantly suppressed in high-Sost cells. Considering that CAII was recently identified as being regulated by Sost/sclerostin and capable of controlling mineral homeostasis, we focused our attention on CAIII. Here, we report that CAIII is highly expressed in osteocytes, is regulated by parathyroid hormone both in vitro and in vivo, and protects osteocytes from oxidative stress.-Shi, C., Uda, Y., Dedic, C., Azab, E., Sun, N., Hussein, A. I., Petty, C. A., Fulzele, K., Mitterberger-Vogt, M. C., Zwerschke, W., Pereira, R., Wang, K., Divieti Pajevic, P. Carbonic anhydrase III protects osteocytes from oxidative stress.
Keywords: PTH; bone homeostasis; sclerostin.
© FASEB.
Conflict of interest statement
This work was partially supported by U.S. National Institutes of Health (NIH)
Figures

References
-
- Balemans W., Ebeling M., Patel N., Van Hul E., Olson P., Dioszegi M., Lacza C., Wuyts W., Van Den Ende J., Willems P., Paes-Alves A. F., Hill S., Bueno M., Ramos F. J., Tacconi P., Dikkers F. G., Stratakis C., Lindpaintner K., Vickery B., Foernzler D., Van Hul W. (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 10, 537–543 - PubMed
-
- Balemans W., Patel N., Ebeling M., Van Hul E., Wuyts W., Lacza C., Dioszegi M., Dikkers F. G., Hildering P., Willems P. J., Verheij J. B., Lindpaintner K., Vickery B., Foernzler D., Van Hul W. (2002) Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J. Med. Genet. 39, 91–97 - PMC - PubMed
-
- Brunkow M. E., Gardner J. C., Van Ness J., Paeper B. W., Kovacevich B. R., Proll S., Skonier J. E., Zhao L., Sabo P. J., Fu Y., Alisch R. S., Gillett L., Colbert T., Tacconi P., Galas D., Hamersma H., Beighton P., Mulligan J. (2001) Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am. J. Hum. Genet. 68, 577–589 - PMC - PubMed
-
- Kim C. A., Honjo R., Bertola D., Albano L., Oliveira L., Jales S., Siqueira J., Castilho A., Balemans W., Piters E., Jennes K., Van Hul W. (2008) A known SOST gene mutation causes sclerosteosis in a familial and an isolated case from Brazilian origin. Genet. Test. 12, 475–479 - PubMed
-
- van Bezooijen R. L., ten Dijke P., Papapoulos S. E., Löwik C. W. (2005) SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev. 16, 319–327 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

