Predictors of responses to immune checkpoint blockade in advanced melanoma
- PMID: 28928380
- PMCID: PMC5605517
- DOI: 10.1038/s41467-017-00608-2
Predictors of responses to immune checkpoint blockade in advanced melanoma
Abstract
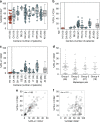
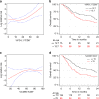
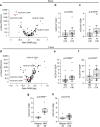
Immune checkpoint blockers (ICB) have become pivotal therapies in the clinical armamentarium against metastatic melanoma (MMel). Given the frequency of immune related adverse events and increasing use of ICB, predictors of response to CTLA-4 and/or PD-1 blockade represent unmet clinical needs. Using a systems biology-based approach to an assessment of 779 paired blood and tumor markers in 37 stage III MMel patients, we analyzed association between blood immune parameters and the functional immune reactivity of tumor-infiltrating cells after ex vivo exposure to ICB. Based on this assay, we retrospectively observed, in eight cohorts enrolling 190 MMel patients treated with ipilimumab, that PD-L1 expression on peripheral T cells was prognostic on overall and progression-free survival. Moreover, detectable CD137 on circulating CD8+ T cells was associated with the disease-free status of resected stage III MMel patients after adjuvant ipilimumab + nivolumab (but not nivolumab alone). These biomarkers should be validated in prospective trials in MMel.The clinical management of metastatic melanoma requires predictors of the response to checkpoint blockade. Here, the authors use immunological assays to identify potential prognostic/predictive biomarkers in circulating blood cells and in tumor-infiltrating lymphocytes from patients with resected stage III melanoma.
Conflict of interest statement
L.C. receives consulting fees from Medimmune and Pfizer and patent/licensing payments from Bristol-Myers Squibb (BMS); L.C. currently receives sponsored research funding from Boehringer Ingelheim, Pfizer and Nextcure. L.F. received research funding from BMS, Merck, Medimmune, Abbvie and Genentech. S.D. is principal investigator, received research grants and congress invitation by BMS. B.W. reports research grants, speaker honoraria, and travel support from MSD/Merck and BMS. I.M. has served as an advisor for Roche-Genentech, BMS, Boehringer Ingelheim, Novartis, Astra-Zeneca Medimmune and Incyte. AME participated on scientific advisory boards for Actelion, Agenus, Bayer, BMS, GlaxoSmithKline (GSK), HalioDx, Incyte, MSD, Nektar, Novartis, Pfizer, and Sanofi. L.Z. is on the Transgene administrative board and Lytix Pharma scientific advisory board and is the main founder of EverImmune. M.J.S. has research agreements with BMS and Corvus Pharmaceuticals and has received patent payments from BMS. A.M.D.G. has honoraria for certified continuing education, and travel supports from BMS, Astra-Zeneca, Roche and MSD. M. Maio has honoraria for certified continuing education, for consulting and advisory roles, research funding and travel supports from BMS, Roche and MSD. A.C.A. is a member of the SAB for Tizona Therapeutics, Potenza Therapeutics, and Idea Pharmaceuticals, which have interests in cancer immunology. A.K. received congress invitation from BMS. C.B. reports honoraria from Lilly, BMS and MSD, serves as advisory roles from Servier and Roche and receives research grants from Roche. B.D. reports BMS board participation, investigator for BMS clinical trials and congress invitation. Y.K. reports ownership interest in a patent regarding serum biomarkers for ipilimumab treatment (WO2016127052.A1). M.H. is a full time employee of Symphogen A/S Denmark. IMS reports honoraria for teaching, consultancy, and advisatory roles from BMS, Roche, Novartis, Genentech, and MSD, research agreement with BMS, Novartis, and Symphogen and co-founder of IO Biotech. R.D. receives research funding from Novartis, MSD, BMS, Roche, GSK and has a consultant or advisory board relationship with Novartis, MSD, BMS, Roche, GSK, Amgen, Takeda, Pierre Fabre. M.B., Y.M., and M.S.F. were supported by the NORFAR program, the Academic Research Office of the Chief Scientist, Ministry of Economy, Israel and collaborated with improdia.co.il. H.S. reports honoraria for teaching, consultancy and advisory boards from BMS, Roche, Merck and Novartis. J.S.W. receives travel grants from and participated to scientific advisory boards of BMS, Merck, Astra-Zeneca, Genentech and EMD Serono. A.M. received consulting honoraria from Roche/Genentech, BMS, Merck (MSD), Astra-Zeneca and participated to scientific advisory boards of Roche/Genentech, Pfizer/Merck serono, Novartis, GSK, eTherna, Kyowa Kirin, Symphogen, Genmab, Amgen, Lytix, Nektar, Seatlle Genetics, Transgene and Flexus Bio. The remaining authors declare no competing financial interests.
Figures




Comment in
-
Predictive immune biomarkers: an unattainable chimera?Cell Mol Immunol. 2018 Aug;15(8):740-742. doi: 10.1038/cmi.2017.162. Epub 2018 Feb 5. Cell Mol Immunol. 2018. PMID: 29400708 Free PMC article. No abstract available.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

