Biomimetically engineered Amphotericin B nano-aggregates circumvent toxicity constraints and treat systemic fungal infection in experimental animals
- PMID: 28928478
- PMCID: PMC5605718
- DOI: 10.1038/s41598-017-11847-0
Biomimetically engineered Amphotericin B nano-aggregates circumvent toxicity constraints and treat systemic fungal infection in experimental animals
Abstract
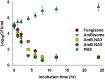
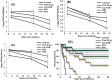
Biomimetic synthesis of nanoparticles offers a convenient and bio friendly approach to fabricate complex structures with sub-nanometer precision from simple precursor components. In the present study, we have synthesized nanoparticles of Amphotericin B (AmB), a potent antifungal agent, using Aloe vera leaf extract. The synthesis of AmB nano-assemblies (AmB-NAs) was established employing spectro-photometric and electron microscopic studies, while their crystalline nature was established by X-ray diffraction. AmB-nano-formulation showed much higher stability in both phosphate buffer saline and serum and exhibit sustained release of parent drug over an extended time period. The as-synthesized AmB-NA possessed significantly less haemolysis as well as nephrotoxicity in the host at par with Ambisome®, a liposomized AmB formulation. Interestingly, the AmB-NAs were more effective in killing various fungal pathogens including Candida spp. and evoked less drug related toxic manifestations in the host as compared to free form of the drug. The data of the present study suggest that biomimetically synthesized AmB-NA circumvent toxicity issues and offer a promising approach to eliminate systemic fungal infections in Balb/C mice.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Aloe vera induced biomimetic assemblage of nucleobase into nanosized particles.PLoS One. 2012;7(3):e32049. doi: 10.1371/journal.pone.0032049. Epub 2012 Mar 5. PLoS One. 2012. PMID: 22403622 Free PMC article.
-
Self-assembled amphotericin B-loaded polyglutamic acid nanoparticles: preparation, characterization and in vitro potential against Candida albicans.Int J Nanomedicine. 2015 Mar 5;10:1769-90. doi: 10.2147/IJN.S63155. eCollection 2015. Int J Nanomedicine. 2015. PMID: 25784804 Free PMC article.
-
Novel oral amphotericin B formulation (iCo-010) remains highly effective against murine systemic candidiasis following exposure to tropical temperature.Drug Dev Ind Pharm. 2015;41(9):1425-30. doi: 10.3109/03639045.2014.954587. Epub 2015 Jul 21. Drug Dev Ind Pharm. 2015. PMID: 25170660
-
Amphotericin B formulations: a comparative review of efficacy and toxicity.Drugs. 2013 Jun;73(9):919-34. doi: 10.1007/s40265-013-0069-4. Drugs. 2013. PMID: 23729001 Review.
-
Amphotericin B and its new formulations: pharmacologic characteristics, clinical efficacy, and tolerability.Transpl Infect Dis. 1999 Dec;1(4):273-83. doi: 10.1034/j.1399-3062.1999.010406.x. Transpl Infect Dis. 1999. PMID: 11428998 Review.
Cited by
-
In vitro investigation of gastrointestinal stability and toxicity of 3-hyrdoxypropionaldehyde (reuterin) produced by Lactobacillus reuteri.Toxicol Rep. 2021 Mar 31;8:740-746. doi: 10.1016/j.toxrep.2021.03.025. eCollection 2021. Toxicol Rep. 2021. PMID: 33868958 Free PMC article.
-
Preparation, Physicochemical Characterization and Anti-Fungal Evaluation of Amphotericin B-Loaded PLGA-PEG-Galactosamine Nanoparticles.Adv Pharm Bull. 2021 Feb;11(2):311-317. doi: 10.34172/apb.2021.044. Epub 2020 Jul 15. Adv Pharm Bull. 2021. PMID: 33880353 Free PMC article.
-
Activity of Auranofin against Multiple Genotypes of Naegleria fowleri and Its Synergistic Effect with Amphotericin B In Vitro.ACS Chem Neurosci. 2020 Aug 19;11(16):2464-2471. doi: 10.1021/acschemneuro.0c00165. Epub 2020 May 26. ACS Chem Neurosci. 2020. PMID: 32392039 Free PMC article.
-
Novel chloroquine loaded curcumin based anionic linear globular dendrimer G2: a metabolomics study on Plasmodium falciparum in vitro using 1H NMR spectroscopy.Parasitology. 2020 Jun;147(7):747-759. doi: 10.1017/S0031182020000372. Epub 2020 Feb 27. Parasitology. 2020. PMID: 32102701 Free PMC article.
-
Activity of Amphotericin B-Loaded Chitosan Nanoparticles against Experimental Cutaneous Leishmaniasis.Molecules. 2020 Sep 2;25(17):4002. doi: 10.3390/molecules25174002. Molecules. 2020. PMID: 32887341 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

