Inhibitory effects of low-intensity pulsed ultrasound sonication on the proliferation of osteosarcoma cells
- PMID: 28928844
- PMCID: PMC5588165
- DOI: 10.3892/ol.2017.6472
Inhibitory effects of low-intensity pulsed ultrasound sonication on the proliferation of osteosarcoma cells
Abstract
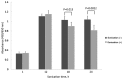
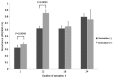
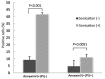
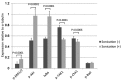
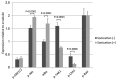
To date, there is limited data on the biological effects of low-intensity pulsed ultrasound (LIPUS) on primary malignant bone tumors. The purpose of the present study was to investigate the antitumor effects of LIPUS on osteosarcoma cells. The effects of LIPUS on cell viability, induction of apoptosis, mitochondrial membrane potential and intracellular signaling molecules in the LM8 osteosarcoma cell line were investigated. LIPUS inhibited cell viability (P=0.0022) and mitochondrial membrane potential (P=0.0019) in LM8 cells. Flow cytometry analysis and terminal deoxynucleotidyl transferase dUTP nick end labeling staining revealed significantly higher numbers of apoptotic (P<0.0001) and necrotic cells (P=0.0091) compared with cells without treatment. LIPUS treatment significantly increased phosphorylated Akt (P<0.0001) and IκBα (P=0.0001) levels, and reduced phosphorylated mitogen-activated protein kinase 7 (P<0.0001) and phosphorylated checkpoint kinase 1 (P=0.0008) levels. These results suggest that LIPUS is a non-invasive adjuvant therapy that is able to inhibit cellular proliferation in osteosarcoma cells.
Keywords: LM8; apoptosis; low-intensity pulsed ultrasound; osteosarcoma; sonication.
Figures





Similar articles
-
Effects of low-intensity pulsed ultrasound on osteosarcoma and cancer cells.Oncol Rep. 2012 Aug;28(2):481-6. doi: 10.3892/or.2012.1816. Epub 2012 May 17. Oncol Rep. 2012. PMID: 22614439
-
Low-intensity pulsed ultrasound promotes the proliferation of human bone mesenchymal stem cells by activating PI3K/AKt signaling pathways.J Cell Biochem. 2019 Sep;120(9):15823-15833. doi: 10.1002/jcb.28853. Epub 2019 May 15. J Cell Biochem. 2019. PMID: 31090943
-
Ultrasound Stimulation of Different Dental Stem Cell Populations: Role of Mitogen-activated Protein Kinase Signaling.J Endod. 2016 Mar;42(3):425-31. doi: 10.1016/j.joen.2015.12.019. Epub 2016 Jan 30. J Endod. 2016. PMID: 26830427
-
Comparison of the in vitro effects of low-level laser therapy and low-intensity pulsed ultrasound therapy on bony cells and stem cells.Prog Biophys Mol Biol. 2018 Mar;133:36-48. doi: 10.1016/j.pbiomolbio.2017.11.001. Epub 2017 Nov 8. Prog Biophys Mol Biol. 2018. PMID: 29126668 Review.
-
Clinical applications of low-intensity pulsed ultrasound and its potential role in urology.Transl Androl Urol. 2016 Apr;5(2):255-66. doi: 10.21037/tau.2016.02.04. Transl Androl Urol. 2016. PMID: 27141455 Free PMC article. Review.
Cited by
-
p38 MAPK signaling is a key mediator for low-intensity pulsed ultrasound (LIPUS) in cultured human omental adipose-derived mesenchymal stem cells.Am J Transl Res. 2019 Jan 15;11(1):418-429. eCollection 2019. Am J Transl Res. 2019. PMID: 30787998 Free PMC article.
-
Progression in low-intensity ultrasound-induced tumor radiosensitization.Cancer Med. 2024 Jul;13(13):e7332. doi: 10.1002/cam4.7332. Cancer Med. 2024. PMID: 38967145 Free PMC article. Review.
-
Acoustic technologies for the orchestration of cellular functions for therapeutic applications.Sci Adv. 2025 Jul 18;11(29):eadu4759. doi: 10.1126/sciadv.adu4759. Epub 2025 Jul 18. Sci Adv. 2025. PMID: 40680134 Free PMC article. Review.
-
Adjuvant Biophysical Therapies in Osteosarcoma.Cancers (Basel). 2019 Mar 12;11(3):348. doi: 10.3390/cancers11030348. Cancers (Basel). 2019. PMID: 30871044 Free PMC article. Review.
-
The reversal of MRP1 expression induced by low-frequency and low-intensity ultrasound and curcumin mediated by VEGF in brain glioma.Onco Targets Ther. 2019 May 13;12:3581-3593. doi: 10.2147/OTT.S195205. eCollection 2019. Onco Targets Ther. 2019. PMID: 31190861 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
