18F-FDG PET/CT as an Indicator of Survival in Ewing Sarcoma of Bone
- PMID: 28928879
- PMCID: PMC5604439
- DOI: 10.7150/jca.20077
18F-FDG PET/CT as an Indicator of Survival in Ewing Sarcoma of Bone
Abstract
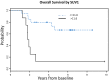
Objective: The existing literature of 18 F-FDG PET/CT in Ewing sarcoma investigates mixed populations of patients with both soft tissue and bone primary tumors. The aim of our study was to evaluate whether the maximum standardized uptake value (SUVmax) obtained with 18F-FDG PET/CT before and after induction chemotherapy can be used as an indicator of survival in patients with Ewing sarcoma originating exclusively in the skeleton. Materials and Methods: A retrospective database search from 2004-2011 identified 28 patients who underwent 18 F-FDG PET/CT before (SUV1, n= 28) and after (SUV2, n=23) induction chemotherapy. Mean follow up was 3.3 years and median follow up for survivors was 6.3 years (range: 2.6-9.8 years). Multivariate and univariate Cox proportional hazard model was used to assess for correlation of SUV1, SUV2, and the change in SUVmax with overall survival (OS) and progression-free survival (PFS). Results: Mean SUVmax was 10.74 before (SUV1) and after 4.11 (SUV2) induction chemotherapy. High SUV1 (HR = 1.05, 95% CI: 1.0-1.1, P = 0.01) and SUV2 (HR =1.2, 95% CI: 1.0-1.4, P = 0.01) were associated with worse OS. A cut off point of 11.6 was identified for SUV1. SUV1 higher than 11.6 had significantly worse OS (HR = 5.71, 95% CI: 1.85 - 17.61, P = 0.003) and PFS (HR = 3.16, 95% CI: 1.13 - 8.79, P = 0.03, P < 0.05 is significant). Conclusion: 18F-FDG PET/CT can be used as a prognostic indicator for survival in primary Ewing sarcoma of bone.
Keywords: Ewing sarcoma; FDG PET/CT; Overall survival; Progression free survival.; SUV.
Conflict of interest statement
Competing Interests: Our third co-author is a consultant for Sage Health Management Solutions.
Figures


Similar articles
-
The role of FDG PET/CT in patients treated with neoadjuvant chemotherapy for localized bone sarcomas.Eur J Nucl Med Mol Imaging. 2017 Feb;44(2):215-223. doi: 10.1007/s00259-016-3509-z. Epub 2016 Sep 20. Eur J Nucl Med Mol Imaging. 2017. PMID: 27645694 Free PMC article. Clinical Trial.
-
[18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors.J Clin Oncol. 2005 Dec 1;23(34):8828-34. doi: 10.1200/JCO.2005.01.7079. J Clin Oncol. 2005. PMID: 16314643
-
Positron emission tomography (18)F-fluorodeoxyglucose uptake and prognosis in patients with bone and soft tissue sarcoma: A meta-analysis.Eur J Surg Oncol. 2016 Aug;42(8):1103-14. doi: 10.1016/j.ejso.2016.04.056. Epub 2016 May 3. Eur J Surg Oncol. 2016. PMID: 27189833 Review.
-
Assessment of Chemotherapy Response Using FDG-PET in Pediatric Bone Tumors: A Single Institution Experience.Cancer Res Treat. 2011 Sep;43(3):170-5. doi: 10.4143/crt.2011.43.3.170. Epub 2011 Sep 30. Cancer Res Treat. 2011. PMID: 22022294 Free PMC article.
-
The Role of 18F-FDG-PET/CT in Pediatric Sarcoma.Semin Nucl Med. 2017 May;47(3):229-241. doi: 10.1053/j.semnuclmed.2016.12.004. Epub 2017 Jan 18. Semin Nucl Med. 2017. PMID: 28417853 Review.
Cited by
-
When SUV Matters: FDG PET/CT at Baseline Correlates with Survival in Soft Tissue and Ewing Sarcoma.Life (Basel). 2021 Aug 24;11(9):869. doi: 10.3390/life11090869. Life (Basel). 2021. PMID: 34575018 Free PMC article.
-
The role of positron emission tomography in the evaluation and management of musculoskeletal lesions-a narrative review.Ann Jt. 2025 Jan 21;10:8. doi: 10.21037/aoj-24-26. eCollection 2025. Ann Jt. 2025. PMID: 39981426 Free PMC article. Review.
-
[18F]FDG PET/CT quantitative parameters for the prediction of histological response to induction chemotherapy and clinical outcome in patients with localised bone and soft-tissue Ewing sarcoma.Eur Radiol. 2021 Sep;31(9):7012-7021. doi: 10.1007/s00330-021-07841-w. Epub 2021 Mar 13. Eur Radiol. 2021. PMID: 33715090
-
Correlation of Transcriptomics and FDG-PET SUVmax Indicates Reciprocal Expression of Stemness-Related Transcription Factor and Neuropeptide Signaling Pathways in Glucose Metabolism of Ewing Sarcoma.Cancers (Basel). 2022 Dec 5;14(23):5999. doi: 10.3390/cancers14235999. Cancers (Basel). 2022. PMID: 36497479 Free PMC article.
-
What and how should we measure in paediatric oncology FDG-PET/CT? Comparison of commonly used SUV metrics for differentiation between paediatric tumours.EJNMMI Res. 2019 Dec 23;9(1):115. doi: 10.1186/s13550-019-0577-7. EJNMMI Res. 2019. PMID: 31872312 Free PMC article.
References
-
- Ludwig JA. Ewing sarcoma: historical perspectives, current state-of-the-art, and opportunities for targeted therapy in the future. Current opinion in oncology. 2008;20:412–8. - PubMed
-
- Herzog CE. Overview of sarcomas in the adolescent and young adult population. J Pediatr Hematol Oncol. 2005;27:215–8. - PubMed
-
- Unni KK, Inwards CY. Ewing Tumor. Dahlin's Bone Tumors: General Aspects and Data on 10,165 Cases. Sixth ed: Wollters Kluwer Health/Lippincott Williams & Wilkins; 2010. pp. 211–24.
-
- Shinichiro Ushigome RM, Poul Sorensen. Ewing sarcoma / Primitive neuroectodermal tumour (PNET) In: Christopher Fletcher KU, Fredrik Mertens, editors. World Health Organization classification of tumours: pathology and genetics of tumours of soft tissue and bone. Lyon: IARCPress; 2002. pp. 298–300.
-
- Esiashvili N, Goodman M, Marcus RB Jr. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol. 2008;30:425–30. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

