In Surgical Colon Cancer Patients Extended-Duration Thromboprophylaxis (30 days) with the Highest Dose of Tinzaparin (4,500 IU s.c./q.d.) Normalizes the Postoperative VEGF Levels
- PMID: 28928880
- PMCID: PMC5604440
- DOI: 10.7150/jca.20107
In Surgical Colon Cancer Patients Extended-Duration Thromboprophylaxis (30 days) with the Highest Dose of Tinzaparin (4,500 IU s.c./q.d.) Normalizes the Postoperative VEGF Levels
Abstract
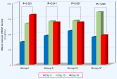
Background/Purpose: In colon cancer (CC) patients preoperative (pre-op) levels of VEGF-A165 (VEGF) is a strong predictor for disease recurrence. Elevated postoperative (post-op) VEGF levels could have undesirable effects by enhancing tumor growth and metastasis formation. It has been suggested that thromboprophylaxis with a Low Molecular Weight Heparin (LMWH) in surgical cancer patients, further to thromboembolic protection, may exert some anti-neoplastic properties, as well. The aim of our study was to assess the potential impact of the LMWH Tinzaparin (Innohep® - Leo Pharma, Copenhagen, Denmark), given at different doses and for different perioperative (peri-op) periods, upon the post-op variability of serum VEGF levels in surgical CC patients. Methods: A total of 54 consecutive CC patients who underwent a curative resection were randomized in four groups according to their peri-op thromboprophylaxis scheme, which was based on administrating Tinzaparin in different doses and at different periods, as follows: group I: 3,500 IU for 10 days, group II: 3,500 IU for 30 days, group III: 4,500 IU for 10 days and group IV: 4,500 IU for 30 days. Serum VEGF concentrations were evaluated on the pre-op day (Day 0) and on the 10th and 30th post-op days (Day 10 and Day 30, respectively). For statistical analyses the mixed design ANOVA was used. P < 0.05 was considered significant. Results: On Day 0, VEGF didn't differ between groups I, II, III and IV (p>0.05, for every comparison). On Day 10, VEGF was increased in all groups. Between Day 10 and Day 30, VEGF remained stable in groups I (p=0.031) and II (p=1.000) and increased significantly in group III (p=0.005). On the contrary, VEGF decreased significantly in group IV (p<0.001). The most remarkable finding was observed when we compared VEGF between Day 0 and Day 30: while in groups I, II and III, VEGF remained significantly higher compared to Day 0 (p<0.001, p=0.041 and p<0.001, respectively), on the contrary, in group IV (extended-duration with the highest dose of 4,500 IU of tinzaparin) it was comparable to Day 0 (p=1.000). Conclusions: In surgical CC patients only the recommended thromboprophylaxis scheme with the highest prophylactic dose of Tinzaparin (4,500 IU) for extended-duration (30 days) normalizes VEGF levels at the end of the first post-op month by reducing them to the pre-op levels.
Keywords: VEGF; angiogenesis.; colon cancer; thromboprophylaxis; tinzaparin.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Efficacy and safety of extended duration to perioperative thromboprophylaxis with low molecular weight heparin on disease-free survival after surgical resection of colorectal cancer (PERIOP-01): multicentre, open label, randomised controlled trial.BMJ. 2022 Sep 13;378:e071375. doi: 10.1136/bmj-2022-071375. BMJ. 2022. PMID: 36100263 Free PMC article. Clinical Trial.
-
Extended thromboprophylaxis post gynaecological cancer surgery; the effect of weight adjusted and fixed dose LMWH (Tinzaparin).Thromb Res. 2021 Nov;207:25-32. doi: 10.1016/j.thromres.2021.08.027. Epub 2021 Sep 7. Thromb Res. 2021. PMID: 34530386
-
Elderly patients treated with tinzaparin (Innohep) administered once daily (175 anti-Xa IU/kg): anti-Xa and anti-IIa activities over 10 days.Thromb Haemost. 2000 Nov;84(5):800-4. Thromb Haemost. 2000. PMID: 11127859
-
Tinzaparin sodium: a review of its pharmacology and clinical use in the prophylaxis and treatment of thromboembolic disease.Drugs. 2004;64(13):1479-502. doi: 10.2165/00003495-200464130-00006. Drugs. 2004. PMID: 15212562 Review.
-
Bemiparin: a review of its use in the prevention of venous thromboembolism and treatment of deep vein thrombosis.Drugs. 2003;63(21):2357-77. doi: 10.2165/00003495-200363210-00009. Drugs. 2003. PMID: 14524738 Review.
Cited by
-
The effect of tinzaparin on biomarkers in FIGO stages III-IV ovarian cancer patients undergoing neoadjuvant chemotherapy - the TABANETOC trial: study protocol for a randomized clinical multicenter trial.Acta Oncol. 2024 Jul 22;63:581-585. doi: 10.2340/1651-226X.2024.40207. Acta Oncol. 2024. PMID: 39037076 Free PMC article.
-
Cancer-Associated Thrombosis: Not All Low-Molecular-Weight Heparins Are the Same, Focus on Tinzaparin, A Narrative Review.Int J Clin Pract. 2022 Jul 19;2022:2582923. doi: 10.1155/2022/2582923. eCollection 2022. Int J Clin Pract. 2022. PMID: 35936060 Free PMC article. Review.
-
The Antineoplastic Effect of Heparin on Colorectal Cancer: A Review of the Literature.J Clin Med. 2023 Nov 19;12(22):7173. doi: 10.3390/jcm12227173. J Clin Med. 2023. PMID: 38002785 Free PMC article. Review.
References
-
- American Cancer Society USA. Key Statistics for Colorectal Cancer. https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-st...; Accessed 14.02.17.
-
- Wieder R. Insurgent micrometastases: sleeper cells and harboring the enemy. J Surg Oncol. 2005;89(4):207–210. - PubMed
-
- Li J, Jiang E, Wang X, Shangguan AJ, Zhang L, Yu Z. Dormant cells: the original cause of tumor recurrence and metastasis. Cell Biochem Biophys. 2015;72(2):317–320. - PubMed
-
- Udagawa T. Tumor dormancy of primary and secondary cancers. APMIS. 2008;116(7-8):615–28. - PubMed
-
- Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: Balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nature Medicine. 1995;1:149–153. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous