The relationship between mucosal immunity, nasopharyngeal carriage, asymptomatic transmission and the resurgence of Bordetella pertussis
- PMID: 28928960
- PMCID: PMC5580413
- DOI: 10.12688/f1000research.11654.1
The relationship between mucosal immunity, nasopharyngeal carriage, asymptomatic transmission and the resurgence of Bordetella pertussis
Abstract
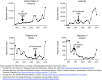
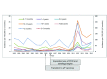
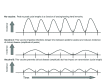
The incidence of whooping cough in the US has been rising slowly since the 1970s, but the pace of this has accelerated sharply since acellular pertussis vaccines replaced the earlier whole cell vaccines in the late 1990s. A similar trend occurred in many other countries, including the UK, Canada, Australia, Ireland, and Spain, following the switch to acellular vaccines. The key question is why. Two leading theories (short duration of protective immunologic persistence and evolutionary shifts in the pathogen to evade the vaccine) explain some but not all of these shifts, suggesting that other factors may also be important. In this synthesis, we argue that sterilizing mucosal immunity that blocks or abbreviates the duration of nasopharyngeal carriage of Bordetella pertussis and impedes person-to-person transmission (including between asymptomatically infected individuals) is a critical factor in this dynamic. Moreover, we argue that the ability to induce such mucosal immunity is fundamentally what distinguishes whole cell and acellular pertussis vaccines and may be pivotal to understanding much of the resurgence of this disease in many countries that adopted acellular vaccines. Additionally, we offer the hypothesis that observed herd effects generated by acellular vaccines may reflect a modification of disease presentation leading to reduced potential for transmission by those already infected, as opposed to inducing resistance to infection among those who have been exposed.
Keywords: acellular pertussis vaccine; bordetella pertussis; whooping cough.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.No competing interests were disclosed.No competing interests were disclosed.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

