Surgical Treatment of Retrosternal Goitre
- PMID: 28929066
- PMCID: PMC5581776
- DOI: 10.1007/s12070-017-1151-0
Surgical Treatment of Retrosternal Goitre
Abstract
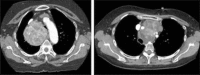
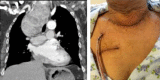
This study aims to evaluate surgical approaches to the management of retrosternal goitre. Between 2004 and 2014, 35 patients (eight males; mean age 67.4 ± 10.9 years) with retrosternal goitre (mainly right-sided in 9, left-sided in 14 and bilateral in 12) underwent surgery. A palpable neck mass was found in 11 (31.4%), stridor in 10 (28.6%) and thyrotoxicosis in 4 (11.4%) cases. 4 (11.4%) patients were asymptomatic. Tracheal compression was detected radiologically in 27 (77.2%) patients with deviation in 18 (51.4%). A collar incision was performed in 34 patients, 6 (17.1%) of whom required additional sternotomy, 1 (2.9%) was assisted by an anterior mediastinotomy. 1 (2.9%) had a right lateral thoracotomy. There was no operative mortality. Transient vocal changes occurred in 3 (8.6%) patients, recurrent laryngeal nerve palsy in 3, atrial fibrillation in 2, and wound complications in 2 (5.7%). Hospital stay ranged from 2 to 12 days (5.5 ± 2.0). Multinodular goitre was found in 33 patients, diffuse goitre in 1 and ectopic thyroid in 1. The average vertical length of goitres in the collar incision group was 7.6 cm compared to 10.6 cm in the sternotomy group. The average weight of specimens was 156.3 g in patients with collar incisions and 307.5 g in the sternotomy group. Removal of retrosternal goitre is more commonly performed via a cervical collar incision with mandatory availability of sternotomy. Radiological measurement of craniocaudal length may predict the risk of sternotomy. Surgical outcomes are not affected by surgical approach.
Keywords: Goitre; RSG; Retrosternal goitre; Sternotomy; Substernal.
Conflict of interest statement
Conflict of interest
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Figures
References
-
- Fan Q, Gong K, Zhu B, Zhang NW. Experience of managing substernal goitre by totally endoscopic procedure. Beijing Da Xue Xue Bao. 2014;46:488–491. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
