Current pesticide dietary risk assessment in light of comparable animal study NOAELs after chronic and short-termed exposure durations
- PMID: 28929275
- PMCID: PMC5773667
- DOI: 10.1007/s00204-017-2052-4
Current pesticide dietary risk assessment in light of comparable animal study NOAELs after chronic and short-termed exposure durations
Abstract
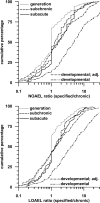
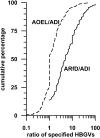
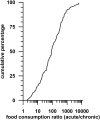
Dietary risk assessment (DRA) of pesticides includes the estimation of chronic and acute exposures from crop residues, but assesses acute exposures only for pesticides with an acute reference dose (ARfD). Acute estimation uses high percentiles of food consumption surveys which are considerably higher than per capita lifetime averaged food consumption values which are used for chronic estimations. Assessing acute risks only for pesticides with an ARfD tacitly assumes that chronic risk assessment covers also intermittent occurring exposures which could significantly exceed chronic estimates. The present investigation conducted on 2200 rat studies from 436 pesticides provides evidence demonstrating that pesticides with and without ARfD have no-observed-adverse-effect levels (NOAELs) which remain statistically unchanged in developmental, subacute, subchronic, reproductive and chronic toxicity studies covering exposure durations between 2 and 104 weeks. DRA of pesticides without ARfD needs reconsideration in light of equally high toxic dose levels after short- and long-term exposures, suggesting that intermittent exposures could be toxic, if they repeatedly exceed the acceptable chronic daily intake (ADI; conceptually the human counterpart of chronic animal NOAEL). As such risks are currently not assessed for pesticides without ARfD, the current DRA concept, which automatically presumes the use of low chronic exposure estimates entirely covers the risks of not acutely toxic pesticides, needs reconsideration. Furthermore, risks to intermittent occurring high exposures are probably also insufficiently assessed for pesticides where the ARfD is significantly higher than the ADI. As an example, the maximum residue limit for bifenazate in peaches is discussed.
Keywords: Dietary risk assessment; Intermittent exposure; NOAEL; Pesticides; Time extrapolation.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The authors declare that this work was not funded by any sources.
Figures

Similar articles
-
Impact of proposed changes in IESTI equations for short-term dietary exposure to pesticides from Australian and Codex perspective.J Environ Sci Health B. 2018 Jun 3;53(6):366-379. doi: 10.1080/03601234.2018.1439812. J Environ Sci Health B. 2018. PMID: 29584575
-
Pesticide residue analysis and its relationship to hazard characterisation (ADI/ARfD) and intake estimations (NEDI/NESTI).Pest Manag Sci. 2002 Oct;58(10):1073-82. doi: 10.1002/ps.544. Pest Manag Sci. 2002. PMID: 12400449 Review.
-
The significance of the subchronic toxicity in the dietary risk assessment of pesticides.Regul Toxicol Pharmacol. 2010 Oct;58(1):72-8. doi: 10.1016/j.yrtph.2010.04.007. Epub 2010 Apr 18. Regul Toxicol Pharmacol. 2010. PMID: 20406660
-
Study parameters influencing NOAEL and LOAEL in toxicity feeding studies for pesticides: exposure duration versus dose decrement, dose spacing, group size and chemical class.Regul Toxicol Pharmacol. 2011 Nov;61(2):243-50. doi: 10.1016/j.yrtph.2011.08.004. Epub 2011 Aug 22. Regul Toxicol Pharmacol. 2011. PMID: 21875639
-
An analysis of the setting of the acute reference dose (ARfD) for pesticides in Europe.Regul Toxicol Pharmacol. 2020 Jun;113:104638. doi: 10.1016/j.yrtph.2020.104638. Epub 2020 Mar 8. Regul Toxicol Pharmacol. 2020. PMID: 32160954 Review.
Cited by
-
Cannabis Contaminants Limit Pharmacological Use of Cannabidiol.Front Pharmacol. 2020 Sep 11;11:571832. doi: 10.3389/fphar.2020.571832. eCollection 2020. Front Pharmacol. 2020. PMID: 33013414 Free PMC article. Review.
-
Dietary risk assessment of drinking water and fish from cultivated wetlands of Ndop.J Water Health. 2024 Jun;22(6):1075-1087. doi: 10.2166/wh.2024.057. Epub 2024 May 28. J Water Health. 2024. PMID: 38935458
-
Chronic oral exposure to pesticides and their consequences on metabolic regulation: role of the microbiota.Eur J Nutr. 2021 Dec;60(8):4131-4149. doi: 10.1007/s00394-021-02548-6. Epub 2021 Apr 10. Eur J Nutr. 2021. PMID: 33837455 Review.
-
Framework for defining pesticide maximum residue levels in feed: applications to cattle and sheep.Pest Manag Sci. 2023 Feb;79(2):748-759. doi: 10.1002/ps.7241. Epub 2022 Nov 2. Pest Manag Sci. 2023. PMID: 36259312 Free PMC article.
-
Toxic Responses Induced at High Doses May Affect Benchmark Doses.Dose Response. 2020 Apr 21;18(2):1559325820919605. doi: 10.1177/1559325820919605. eCollection 2020 Apr-Jun. Dose Response. 2020. PMID: 32341684 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

