O-GlcNAc-Dependent Regulation of Progesterone Receptor Function in Breast Cancer
- PMID: 28929346
- PMCID: PMC5775912
- DOI: 10.1007/s12672-017-0310-9
O-GlcNAc-Dependent Regulation of Progesterone Receptor Function in Breast Cancer
Abstract
Emerging clinical trial data implicate progestins in the development of breast cancer. While the role for the progesterone receptor (PR) in this process remains controversial, it is clear that PR, a steroid-activated nuclear receptor, alters the transcriptional landscape of breast cancer. PR interacts with many different types of proteins, including transcriptional co-activators and co-repressors, transcription factors, nuclear receptors, and proteins that post-translationally modify PR (i.e., kinases and phosphatases). Herein, we identify a novel interaction between PR and O-GlcNAc transferase (OGT), the enzyme that catalyzes the addition of a single N-acetylglucosamine sugar, referred to as O-GlcNAc, to acceptor serines and threonines in target proteins. This interaction between PR and OGT leads to the post-translational modification of PR by O-GlcNAc. Moreover, we show that O-GlcNAcylated PR is more transcriptionally active on PR-target genes, despite the observation that PR messenger RNA and protein levels are decreased when O-GlcNAc levels are high. O-GlcNAcylation in breast cancer is clinically relevant, as we show that O-GlcNAc levels are higher in breast cancer as compared to matched normal tissues, and PR-positive breast cancers have higher levels of OGT. These data predict that under conditions where O-GlcNAc levels are high (breast cancer), PR, through an interaction with the modifying enzyme OGT, will exhibit increased O-GlcNAcylation and potentiated transcriptional activity. Therapeutic strategies aimed at altering cellular O-GlcNAc levels may have profound effects on PR transcriptional activity in breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


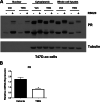
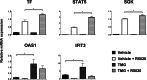
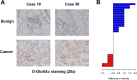

Similar articles
-
Site-specific O-GlcNAcylation of progesterone receptor (PR) supports PR attenuation of interferon stimulated genes (ISGs) and tumor growth in breast cancer.J Biol Chem. 2024 Nov;300(11):107886. doi: 10.1016/j.jbc.2024.107886. Epub 2024 Oct 11. J Biol Chem. 2024. PMID: 39395796 Free PMC article.
-
mTOR/MYC Axis Regulates O-GlcNAc Transferase Expression and O-GlcNAcylation in Breast Cancer.Mol Cancer Res. 2015 May;13(5):923-33. doi: 10.1158/1541-7786.MCR-14-0536. Epub 2015 Jan 30. Mol Cancer Res. 2015. PMID: 25636967 Free PMC article.
-
Changes in O-Linked N-Acetylglucosamine (O-GlcNAc) Homeostasis Activate the p53 Pathway in Ovarian Cancer Cells.J Biol Chem. 2016 Sep 2;291(36):18897-914. doi: 10.1074/jbc.M116.734533. Epub 2016 Jul 11. J Biol Chem. 2016. PMID: 27402830 Free PMC article.
-
Role of O-Linked N-Acetylglucosamine Protein Modification in Cellular (Patho)Physiology.Physiol Rev. 2021 Apr 1;101(2):427-493. doi: 10.1152/physrev.00043.2019. Epub 2020 Jul 30. Physiol Rev. 2021. PMID: 32730113 Free PMC article. Review.
-
The potential role of O-GlcNAc modification in cancer epigenetics.Cell Mol Biol Lett. 2014 Sep;19(3):438-60. doi: 10.2478/s11658-014-0204-6. Epub 2014 Aug 20. Cell Mol Biol Lett. 2014. PMID: 25141978 Free PMC article. Review.
Cited by
-
O-GlcNAcylation Enhances NUSAP1 Stability and Promotes Bladder Cancer Aggressiveness.Onco Targets Ther. 2021 Jan 15;14:445-454. doi: 10.2147/OTT.S258175. eCollection 2021. Onco Targets Ther. 2021. PMID: 33488099 Free PMC article.
-
Metabolic Adaptations in an Endocrine-Related Breast Cancer Mouse Model Unveil Potential Markers of Tumor Response to Hormonal Therapy.Front Oncol. 2022 Mar 1;12:786931. doi: 10.3389/fonc.2022.786931. eCollection 2022. Front Oncol. 2022. PMID: 35299741 Free PMC article.
-
O-GlcNAcylation in Endocrinology: The Sweet Link.Endocrinology. 2025 Apr 22;166(6):bqaf072. doi: 10.1210/endocr/bqaf072. Endocrinology. 2025. PMID: 40209111 Review.
-
Non-targeted metabolomics reveals diagnostic biomarker in the tongue coating of patients with chronic gastritis.J Pharm Biomed Anal. 2019 Sep 10;174:541-551. doi: 10.1016/j.jpba.2019.06.025. Epub 2019 Jun 20. J Pharm Biomed Anal. 2019. PMID: 31255854 Free PMC article.
-
Site-specific O-GlcNAcylation of progesterone receptor (PR) supports PR attenuation of interferon stimulated genes (ISGs) and tumor growth in breast cancer.J Biol Chem. 2024 Nov;300(11):107886. doi: 10.1016/j.jbc.2024.107886. Epub 2024 Oct 11. J Biol Chem. 2024. PMID: 39395796 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

