Laminar-specific encoding of texture elements in rat barrel cortex
- PMID: 28929510
- PMCID: PMC5709323
- DOI: 10.1113/JP274865
Laminar-specific encoding of texture elements in rat barrel cortex
Abstract
Key points: For rats texture discrimination is signalled by the large face whiskers by stick-slip events. Neural encoding of repetitive stick-slip events will be influenced by intrinsic properties of adaptation. We show that texture coding in the barrel cortex is laminar specific and follows a power function. Our results also show layer 2 codes for novel feature elements via robust firing rates and temporal fidelity. We conclude that texture coding relies on a subtle neural ensemble to provide important object information.
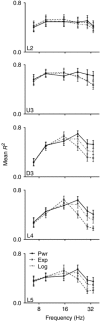
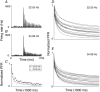
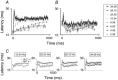
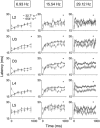
Abstract: Texture discrimination by rats is exquisitely guided by fine-grain mechanical stick-slip motions of the face whiskers as they encounter, stick to and slip past successive texture-defining surface features such as bumps and grooves. Neural encoding of successive stick-slip texture events will be shaped by adaptation, common to all sensory systems, whereby receptor and neural responses to a stimulus are affected by responses to preceding stimuli, allowing resetting to signal novel information. Additionally, when a whisker is actively moved to contact and brush over surfaces, that motion itself generates neural responses that could cause adaptation of responses to subsequent stick-slip events. Nothing is known about encoding in the rat whisker system of stick-slip events defining textures of different grain or the influence of adaptation from whisker protraction or successive texture-defining stick-slip events. Here we recorded responses from halothane-anaesthetized rats in response to texture-defining stimuli applied to passive whiskers. We demonstrate that: across the columnar network of the whisker-recipient barrel cortex, adaptation in response to repetitive stick-slip events is strongest in uppermost layers and equally lower thereafter; neither whisker protraction speed nor stick-slip frequency impede encoding of stick-slip events at rates up to 34.08 Hz; and layer 2 normalizes responses to whisker protraction to resist effects on texture signalling. Thus, within laminar-specific response patterns, barrel cortex reliably encodes texture-defining elements even to high frequencies.
Keywords: barrel cortex; electrophysiology; texture discrimination.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Conflict of interest statement
The authors have no conflicts of interest.
Figures
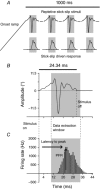
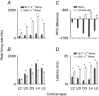


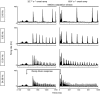





Similar articles
-
Texture coding in the rat whisker system: slip-stick versus differential resonance.PLoS Biol. 2008 Aug 26;6(8):e215. doi: 10.1371/journal.pbio.0060215. PLoS Biol. 2008. PMID: 18752354 Free PMC article.
-
Slip-Based Coding of Local Shape and Texture in Mouse S1.Neuron. 2018 Jan 17;97(2):418-433.e5. doi: 10.1016/j.neuron.2017.12.021. Epub 2018 Jan 4. Neuron. 2018. PMID: 29307709 Free PMC article.
-
Coexisting neuronal coding strategies in the barrel cortex.Cereb Cortex. 2022 Nov 9;32(22):4986-5004. doi: 10.1093/cercor/bhab527. Cereb Cortex. 2022. PMID: 35149866
-
Sensorimotor processing in the rodent barrel cortex.Nat Rev Neurosci. 2019 Sep;20(9):533-546. doi: 10.1038/s41583-019-0200-y. Epub 2019 Jul 31. Nat Rev Neurosci. 2019. PMID: 31367018 Free PMC article. Review.
-
Representation of tactile scenes in the rodent barrel cortex.Neuroscience. 2018 Jan 1;368:81-94. doi: 10.1016/j.neuroscience.2017.08.039. Epub 2017 Aug 23. Neuroscience. 2018. PMID: 28843997 Review.
Cited by
-
Contribution of Interneuron Subtype-Specific GABAergic Signaling to Emergent Sensory Processing in Mouse Somatosensory Whisker Barrel Cortex.Cereb Cortex. 2022 Jun 7;32(12):2538-2554. doi: 10.1093/cercor/bhab363. Cereb Cortex. 2022. PMID: 34613375 Free PMC article.
-
Physiological noise facilitates multiplexed coding of vibrotactile-like signals in somatosensory cortex.Proc Natl Acad Sci U S A. 2022 Sep 13;119(37):e2118163119. doi: 10.1073/pnas.2118163119. Epub 2022 Sep 6. Proc Natl Acad Sci U S A. 2022. PMID: 36067307 Free PMC article.
-
Velocity of conduction between columns and layers in barrel cortex reported by parvalbumin interneurons.Cereb Cortex. 2023 Aug 23;33(17):9917-9926. doi: 10.1093/cercor/bhad254. Cereb Cortex. 2023. PMID: 37415260 Free PMC article.
-
Sensory Adaptation in the Whisker-Mediated Tactile System: Physiology, Theory, and Function.Front Neurosci. 2021 Oct 29;15:770011. doi: 10.3389/fnins.2021.770011. eCollection 2021. Front Neurosci. 2021. PMID: 34776857 Free PMC article. Review.
-
Inter and Intralaminar Excitation of Parvalbumin Interneurons in Mouse Barrel Cortex.bioRxiv [Preprint]. 2023 Jun 19:2023.06.02.543448. doi: 10.1101/2023.06.02.543448. bioRxiv. 2023. Update in: PLoS One. 2024 Jun 13;19(6):e0289901. doi: 10.1371/journal.pone.0289901. PMID: 37398428 Free PMC article. Updated. Preprint.
References
-
- Abdi H (2007). The Bonferonni and Šidák corrections for multiple comparisons In Encyclopedia of Measurement and Statistics, ed. Salkind N. Sage, Thousand Oaks, CA.
-
- Ahissar E, Sosnik R & Haidarliu S (2000). Transformation from temporal to rate coding in a somatosensory thalamocortical pathway. Nature 406, 302–306. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

