Identification of bottlenecks in the accumulation of cyclic fatty acids in camelina seed oil
- PMID: 28929610
- PMCID: PMC5866947
- DOI: 10.1111/pbi.12839
Identification of bottlenecks in the accumulation of cyclic fatty acids in camelina seed oil
Abstract
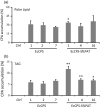
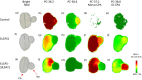
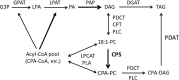
Modified fatty acids (mFA) have diverse uses; for example, cyclopropane fatty acids (CPA) are feedstocks for producing coatings, lubricants, plastics and cosmetics. The expression of mFA-producing enzymes in crop and model plants generally results in lower levels of mFA accumulation than in their natural-occurring source plants. Thus, to further our understanding of metabolic bottlenecks that limit mFA accumulation, we generated transgenic Camelina sativa lines co-expressing Escherichia coli cyclopropane synthase (EcCPS) and Sterculia foetida lysophosphatidic acid acyltransferase (SfLPAT). In contrast to transgenic CPA-accumulating Arabidopsis, CPA accumulation in camelina caused only minor changes in seed weight, germination rate, oil accumulation and seedling development. CPA accumulated to much higher levels in membrane than storage lipids, comprising more than 60% of total fatty acid in both phosphatidylcholine (PC) and phosphatidylethanolamine (PE) versus 26% in diacylglycerol (DAG) and 12% in triacylglycerol (TAG) indicating bottlenecks in the transfer of CPA from PC to DAG and from DAG to TAG. Upon co-expression of SfLPAT with EcCPS, di-CPA-PC increased by ~50% relative to lines expressing EcCPS alone with the di-CPA-PC primarily observed in the embryonic axis and mono-CPA-PC primarily in cotyledon tissue. EcCPS-SfLPAT lines revealed a redistribution of CPA from the sn-1 to sn-2 positions within PC and PE that was associated with a doubling of CPA accumulation in both DAG and TAG. The identification of metabolic bottlenecks in acyl transfer between site of synthesis (phospholipids) and deposition in storage oils (TAGs) lays the foundation for the optimizing CPA accumulation through directed engineering of oil synthesis in target crops.
Keywords: Camelina sativa; cyclopropane fatty acid; lipid metabolism; lipid synthesis; triacylglycerol; unusual fatty acid.
© 2017 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Figures




Similar articles
-
Expression of a Lychee PHOSPHATIDYLCHOLINE:DIACYLGLYCEROL CHOLINEPHOSPHOTRANSFERASE with an Escherichia coli CYCLOPROPANE SYNTHASE Enhances Cyclopropane Fatty Acid Accumulation in Camelina Seeds.Plant Physiol. 2019 Jul;180(3):1351-1361. doi: 10.1104/pp.19.00396. Epub 2019 May 13. Plant Physiol. 2019. PMID: 31123096 Free PMC article.
-
Coexpressing Escherichia coli cyclopropane synthase with Sterculia foetida Lysophosphatidic acid acyltransferase enhances cyclopropane fatty acid accumulation.Plant Physiol. 2014 Jan;164(1):455-65. doi: 10.1104/pp.113.230953. Epub 2013 Nov 7. Plant Physiol. 2014. PMID: 24204024 Free PMC article.
-
Two Acyltransferases Contribute Differently to Linolenic Acid Levels in Seed Oil.Plant Physiol. 2017 Apr;173(4):2081-2095. doi: 10.1104/pp.16.01865. Epub 2017 Feb 24. Plant Physiol. 2017. PMID: 28235891 Free PMC article.
-
Camelina sativa: An ideal platform for the metabolic engineering and field production of industrial lipids.Biochimie. 2016 Jan;120:9-16. doi: 10.1016/j.biochi.2015.06.009. Epub 2015 Jun 21. Biochimie. 2016. PMID: 26107412 Review.
-
Born of frustration: the emergence of Camelina sativa as a platform for lipid biotechnology.Plant Physiol. 2025 Feb 7;197(2):kiaf009. doi: 10.1093/plphys/kiaf009. Plant Physiol. 2025. PMID: 39813144 Free PMC article. Review.
Cited by
-
Triacylglycerol remodeling in Physaria fendleri indicates oil accumulation is dynamic and not a metabolic endpoint.Plant Physiol. 2021 Oct 5;187(2):799-815. doi: 10.1093/plphys/kiab294. Plant Physiol. 2021. PMID: 34608961 Free PMC article.
-
Genetics, Breeding and Genetic Engineering to Improve Cottonseed Oil and Protein: A Review.Front Plant Sci. 2022 Mar 10;13:864850. doi: 10.3389/fpls.2022.864850. eCollection 2022. Front Plant Sci. 2022. PMID: 35360295 Free PMC article. Review.
-
Expression of a Lychee PHOSPHATIDYLCHOLINE:DIACYLGLYCEROL CHOLINEPHOSPHOTRANSFERASE with an Escherichia coli CYCLOPROPANE SYNTHASE Enhances Cyclopropane Fatty Acid Accumulation in Camelina Seeds.Plant Physiol. 2019 Jul;180(3):1351-1361. doi: 10.1104/pp.19.00396. Epub 2019 May 13. Plant Physiol. 2019. PMID: 31123096 Free PMC article.
-
Castor LPCAT and PDAT1A Act in Concert to Promote Transacylation of Hydroxy-Fatty Acid onto Triacylglycerol.Plant Physiol. 2020 Oct;184(2):709-719. doi: 10.1104/pp.20.00691. Epub 2020 Jul 31. Plant Physiol. 2020. PMID: 32737074 Free PMC article.
-
A toolkit for plant lipid engineering: Surveying the efficacies of lipogenic factors for accumulating specialty lipids.Front Plant Sci. 2022 Dec 14;13:1064176. doi: 10.3389/fpls.2022.1064176. eCollection 2022. Front Plant Sci. 2022. PMID: 36589075 Free PMC article.
References
-
- Arroyo‐Caro, J.M. , Chileh, T. , Kazachkov, M. , Zou, J.T. , Alonso, D.L. and Garcia‐Maroto, F. (2013) The multigene family of lysophosphatidate acyltransferase (LPAT)‐related enzymes in Ricinus communis. Cloning and molecular characterization of two LPAT genes that are expressed in castor seeds. Plant Sci. 199, 29–40. - PubMed
-
- Bao, X. , Thelen, J.J. , Bonaventure, G. and Ohlrogge, J.B. (2003) Characterization of cyclopropane fatty‐acid synthase from Sterculia foetida. J. Biol. Chem. 278, 12846–12853. - PubMed
-
- Bates, P.D. (2016) Understanding the control of acyl flux through the lipid metabolic network of plant oil biosynthesis. BBA‐Mol. Cell. Biol. L. 1861, 1214–1225. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

