Heart Repair Using Nanogel-Encapsulated Human Cardiac Stem Cells in Mice and Pigs with Myocardial Infarction
- PMID: 28929735
- PMCID: PMC5656981
- DOI: 10.1021/acsnano.7b01008
Heart Repair Using Nanogel-Encapsulated Human Cardiac Stem Cells in Mice and Pigs with Myocardial Infarction
Abstract
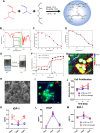
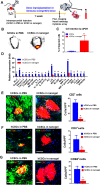
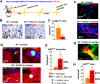
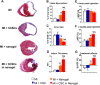
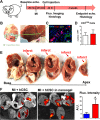
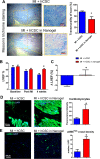
Stem cell transplantation is currently implemented clinically but is limited by low retention and engraftment of transplanted cells and the adverse effects of inflammation and immunoreaction when allogeneic or xenogeneic cells are used. Here, we demonstrate the safety and efficacy of encapsulating human cardiac stem cells (hCSCs) in thermosensitive poly(N-isopropylacrylamine-co-acrylic acid) or P(NIPAM-AA) nanogel in mouse and pig models of myocardial infarction (MI). Unlike xenogeneic hCSCs injected in saline, injection of nanogel-encapsulated hCSCs does not elicit systemic inflammation or local T cell infiltrations in immunocompetent mice. In mice and pigs with acute MI, injection of encapsulated hCSCs preserves cardiac function and reduces scar sizes, whereas injection of hCSCs in saline has an adverse effect on heart healing. In conclusion, thermosensitive nanogels can be used as a stem cell carrier: the porous and convoluted inner structure allows nutrient, oxygen, and secretion diffusion but can prevent the stem cells from being attacked by immune cells.
Keywords: biomaterials; cardiac stem cells; mouse model; myocardial infarction; nanogel; pig model.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Bolli R.; Chugh A. R.; D’Amario D.; Loughran J. H.; Stoddard M. F.; Ikram S.; Beache G. M.; Wagner S. G.; Leri A.; Hosoda T.; et al. Cardiac Stem Cells in Patients with Ischaemic Cardiomyopathy (SCIPIO): Initial Results of a Randomised Phase 1 Trial. Lancet 2011, 378, 1847–57. 10.1016/S0140-6736(11)61590-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

