Host Transcriptional Response to Ebola Virus Infection
- PMID: 28930167
- PMCID: PMC5620561
- DOI: 10.3390/vaccines5030030
Host Transcriptional Response to Ebola Virus Infection
Abstract
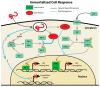
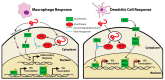
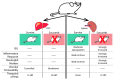
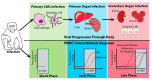
Ebola virus disease (EVD) is a serious illness that causes severe disease in humans and non-human primates (NHPs) and has mortality rates up to 90%. EVD is caused by the Ebolavirus and currently there are no licensed therapeutics or vaccines to treat EVD. Due to its high mortality rates and potential as a bioterrorist weapon, a better understanding of the disease is of high priority. Multiparametric analysis techniques allow for a more complete understanding of a disease and the host response. Analysis of RNA species present in a sample can lead to a greater understanding of activation or suppression of different states of the immune response. Transcriptomic analyses such as microarrays and RNA-Sequencing (RNA-Seq) have been important tools to better understand the global gene expression response to EVD. In this review, we outline the current knowledge gained by transcriptomic analysis of EVD.
Keywords: Ebola virus disease; RNA-Seq; host response; immune response; microarray; transcriptomic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Characterization of Ebola Virus Mucosal Challenge Routes in Cynomolgus Macaques.J Virol. 2023 May 31;97(5):e0188822. doi: 10.1128/jvi.01888-22. Epub 2023 Mar 28. J Virol. 2023. PMID: 36975793 Free PMC article.
-
Distinct Immunogenicity and Efficacy of Poxvirus-Based Vaccine Candidates against Ebola Virus Expressing GP and VP40 Proteins.J Virol. 2018 May 14;92(11):e00363-18. doi: 10.1128/JVI.00363-18. Print 2018 Jun 1. J Virol. 2018. PMID: 29514907 Free PMC article.
-
Advances in Designing and Developing Vaccines, Drugs, and Therapies to Counter Ebola Virus.Front Immunol. 2018 Aug 10;9:1803. doi: 10.3389/fimmu.2018.01803. eCollection 2018. Front Immunol. 2018. PMID: 30147687 Free PMC article. Review.
-
Ebolavirus Vaccines: Progress in the Fight Against Ebola Virus Disease.Cell Physiol Biochem. 2015;37(5):1641-58. doi: 10.1159/000438531. Epub 2015 Nov 5. Cell Physiol Biochem. 2015. PMID: 26535889 Review.
-
Investigating the Cellular Transcriptomic Response Induced by the Makona Variant of Ebola Virus in Differentiated THP-1 Cells.Viruses. 2019 Nov 4;11(11):1023. doi: 10.3390/v11111023. Viruses. 2019. PMID: 31689981 Free PMC article.
Cited by
-
Natural history of Ebola virus disease in rhesus monkeys shows viral variant emergence dynamics and tissue-specific host responses.Cell Genom. 2023 Nov 21;3(12):100440. doi: 10.1016/j.xgen.2023.100440. eCollection 2023 Dec 13. Cell Genom. 2023. PMID: 38169842 Free PMC article.
-
Comparative Transcriptomics in Ebola Makona-Infected Ferrets, Nonhuman Primates, and Humans.J Infect Dis. 2018 Nov 22;218(suppl_5):S486-S495. doi: 10.1093/infdis/jiy455. J Infect Dis. 2018. PMID: 30476250 Free PMC article.
-
Quantification of Viral and Host Biomarkers in the Liver of Rhesus Macaques: A Longitudinal Study of Zaire Ebolavirus Strain Kikwit (EBOV/Kik).Am J Pathol. 2020 Jul;190(7):1449-1460. doi: 10.1016/j.ajpath.2020.03.003. Epub 2020 Apr 8. Am J Pathol. 2020. PMID: 32275904 Free PMC article.
-
Expansion of Single Cell Transcriptomics Data of SARS-CoV Infection in Human Bronchial Epithelial Cells to COVID-19.Biol Proced Online. 2020 Jul 23;22:16. doi: 10.1186/s12575-020-00127-3. eCollection 2020. Biol Proced Online. 2020. PMID: 32754004 Free PMC article.
-
Transcriptomic Analysis Reveals Host miRNAs Correlated with Immune Gene Dysregulation during Fatal Disease Progression in the Ebola Virus Cynomolgus Macaque Disease Model.Microorganisms. 2021 Mar 23;9(3):665. doi: 10.3390/microorganisms9030665. Microorganisms. 2021. PMID: 33806942 Free PMC article.
References
-
- CDC 2014 Ebola Outbreak in West Africa: Case Counts. Centers for Disease Control and Prevention. [(accessed on 1 June 2017)]; Available online: http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html.
-
- Lüdtke A., Ruibal P., Wozniak D.M., Pallasch E., Wurr S., Bockholt S., Gómez-Medina S., Qiu X., Kobinger G.P., Rodríguez E., et al. Ebola virus infection kinetics in chimeric mice reveal a key role of T cells as barriers for virus dissemination. Sci. Rep. 2017 doi: 10.1038/srep43776. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

