Sensitive Genotyping of Foodborne-Associated Human Noroviruses and Hepatitis A Virus Using an Array-Based Platform
- PMID: 28930175
- PMCID: PMC5621023
- DOI: 10.3390/s17092157
Sensitive Genotyping of Foodborne-Associated Human Noroviruses and Hepatitis A Virus Using an Array-Based Platform
Abstract
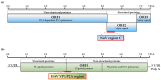
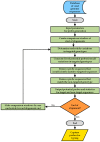
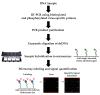
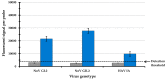
Human noroviruses (NoV) are the leading cause of human gastroenteritis in populations of all ages and are linked to most of the foodborne outbreaks worldwide. Hepatitis A virus (HAV) is another important foodborne enteric virus and is considered the most common agent causing acute liver disease worldwide. In the present study, a focused, low-density DNA microarray was developed and validated for the simultaneous identification of foodborne-associated genotypes of NoV and HAV. By employing a novel algorithm, capture probes were designed to target variable genomic regions commonly used for typing these foodborne viruses. Validation results showed that probe signals, specific for the tested NoV or HAV genotypes, were on average 200-times or 38-times higher than those detected for non-targeted genotypes, respectively. To improve the analytical sensitivity of this method, a 12-mer oligonucleotide spacer sequence was added to the capture probes and resulted in a detection threshold of less than 10 cRNA transcripts. These findings have indicated that this array-based typing sensor has the accuracy and sensitivity for identifying NoV and HAV genotypic profiles predominantly linked to food poisoning. The implementation of this typing sensor would thus provide highly relevant and valuable information for use in surveillance and outbreak attribution.
Keywords: food safety; foodborne pathogen; genotyping; hepatitis A virus; microarray; norovirus; pathogen detection; viruses.
Conflict of interest statement
B.Q., B.G.L. and J.C.Y. declare no conflict of interest. T.J.M., P.K.H. and M.S. are employed by Arrayit Corporation, Sunnyvale, CA, USA.
Figures
Similar articles
-
Norovirus genotype profiles associated with foodborne transmission, 1999-2012.Emerg Infect Dis. 2015 Apr;21(4):592-9. doi: 10.3201/eid2104.141073. Emerg Infect Dis. 2015. PMID: 25811368 Free PMC article.
-
Foodborne viruses: an emerging problem.Int J Food Microbiol. 2004 Jan 1;90(1):23-41. doi: 10.1016/s0168-1605(03)00169-7. Int J Food Microbiol. 2004. PMID: 14672828 Free PMC article. Review.
-
Use of sequence analysis of the P2 domain for characterization of norovirus strains causing a large multistate outbreak of norovirus gastroenteritis in Germany 2012.Int J Med Microbiol. 2015 Oct;305(7):612-8. doi: 10.1016/j.ijmm.2015.08.010. Epub 2015 Aug 21. Int J Med Microbiol. 2015. PMID: 26341330
-
Estimating risk associated with human norovirus and hepatitis A virus in fresh Australian leafy greens and berries at retail.Int J Food Microbiol. 2019 Nov 15;309:108327. doi: 10.1016/j.ijfoodmicro.2019.108327. Epub 2019 Aug 26. Int J Food Microbiol. 2019. PMID: 31493567
-
Foodborne transmission of hepatitis A and hepatitis E viruses: A literature review.Int J Food Microbiol. 2021 Jan 2;338:108986. doi: 10.1016/j.ijfoodmicro.2020.108986. Epub 2020 Nov 19. Int J Food Microbiol. 2021. PMID: 33257099 Review.
Cited by
-
Molecular epidemiology of hepatitis A outbreaks in two districts in Indonesia in 2018: Same subtype, but different strains.Biomed Rep. 2020 Feb;12(2):51-58. doi: 10.3892/br.2019.1261. Epub 2019 Nov 27. Biomed Rep. 2020. PMID: 31929874 Free PMC article.
-
Lithographic Processes for the Scalable Fabrication of Micro- and Nanostructures for Biochips and Biosensors.ACS Sens. 2021 Jun 25;6(6):2002-2024. doi: 10.1021/acssensors.0c02704. Epub 2021 Apr 8. ACS Sens. 2021. PMID: 33829765 Free PMC article. Review.
-
A Survey of Analytical Techniques for Noroviruses.Foods. 2020 Mar 10;9(3):318. doi: 10.3390/foods9030318. Foods. 2020. PMID: 32164213 Free PMC article. Review.
-
Advances in Diagnostic Approaches for Viral Etiologies of Diarrhea: From the Lab to the Field.Front Microbiol. 2019 Sep 13;10:1957. doi: 10.3389/fmicb.2019.01957. eCollection 2019. Front Microbiol. 2019. PMID: 31608017 Free PMC article. Review.
References
-
- Green K.Y. Caliciviridae: The noroviruses. In: Knipe D.M., Howley P.M., Cohen J.I., Griffin D.E., Lamb R.A., Martin M.A., Racaniello V.R., Roizman B., editors. Fields Virology. 6th ed. Volume 1. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2013. pp. 582–608.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

