Substituting Sodium Hydrosulfite with Sodium Metabisulfite Improves Long-Term Stability of a Distributable Paper-Based Test Kit for Point-of-Care Screening for Sickle Cell Anemia
- PMID: 28930183
- PMCID: PMC5618045
- DOI: 10.3390/bios7030039
Substituting Sodium Hydrosulfite with Sodium Metabisulfite Improves Long-Term Stability of a Distributable Paper-Based Test Kit for Point-of-Care Screening for Sickle Cell Anemia
Abstract
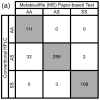
Sickle cell anemia (SCA) is a genetic blood disorder that is particularly lethal in early childhood. Universal newborn screening programs and subsequent early treatment are known to drastically reduce under-five SCA mortality. However, in resource-limited settings, cost and infrastructure constraints limit the effectiveness of laboratory-based SCA screening programs. To address this limitation our laboratory previously developed a low-cost, equipment-free, point-of-care, paper-based SCA test. Here, we improved the stability and performance of the test by replacing sodium hydrosulfite (HS), a key reducing agent in the hemoglobin solubility buffer which is not stable in aqueous solutions, with sodium metabisulfite (MS). The MS formulation of the test was compared to the HS formulation in a laboratory setting by inexperienced users (n = 3), to determine visual limit of detection (LOD), readout time, diagnostic accuracy, intra- and inter-observer agreement, and shelf life. The MS test was found to have a 10% sickle hemoglobin LOD, 21-min readout time, 97.3% sensitivity and 99.5% specificity for SCA, almost perfect intra- and inter-observer agreement, at least 24 weeks of shelf stability at room temperature, and could be packaged into a self-contained, distributable test kits comprised of off-the-shelf disposable components and food-grade reagents with a total cost of only $0.21 (USD).
Keywords: paper-based diagnostics; point-of-care screening; sickle cell anemia.
Conflict of interest statement
S.S.S. and N.Z.P. are inventors on a utility PCT application “Paper based diagnostic test” (PCT/US2012/064856, 11/13/2012) claiming priority benefit of US 61/692,994 (8/24/2012) and US 61/558,009 (11/10/2011) []. S.S.S. is a part-owner of Halcyon Biomedical Incorporated, a company that may benefit from commercialization of the paper-based SCA test(s). All other authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

