Oligonol, a Low-Molecular Weight Polyphenol Derived from Lychee, Alleviates Muscle Loss in Diabetes by Suppressing Atrogin-1 and MuRF1
- PMID: 28930190
- PMCID: PMC5622800
- DOI: 10.3390/nu9091040
Oligonol, a Low-Molecular Weight Polyphenol Derived from Lychee, Alleviates Muscle Loss in Diabetes by Suppressing Atrogin-1 and MuRF1
Abstract
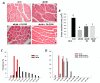
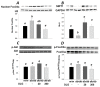
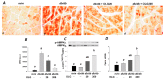
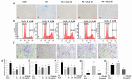
Stimulation of the ubiquitin-proteasome pathway-especially E3 ubiquitin ligases Atrogin-1 and MuRF1-is associated with muscle loss in diabetes. Elevated lipid metabolites impair myogenesis. Oligonol, a low molecular weight polyphenol derived from lychee, exhibited anti-diabetic and anti-obesity properties, suggesting it could be a proper supplement for attenuating muscle loss. Dietary (10 weeks) oligonol supplementation (20 or 200 mg/kg diet) on the skeletal muscle loss was investigated in diabetic db/db mice. Transcription factors NF-κB and FoxO3a involved in regulation of Atrogin-1 and MuRF1 were also investigated. Attenuation of muscle loss by oligonol (both doses) was associated with down-regulation of Atrogin-1 and MuRF1 gene expression. Oligonol supplementation decreased NF-κB expression in the nuclear fraction compared with db/db mice without oligonol supplement. Upregulation of sirtuin1 (SIRT1) expression prevented FoxO3a nuclear localization in db/db mice supplemented with oligonol. Marked increases in AMPKα activity and Ppara mRNA expression leading to lower lipid accumulation by oligonol provided additional benefits for attenuating muscle loss. Oligonol limited palmitate-induced senescent phenotype and cell cycle arrest and suppressed Atrogin-1 and MuRF1 mRNA expression in palmitate-treated C2C12 muscle cells, thus contributing to improving the impaired myotube formation. In conclusion, oligonol-mediated downregulation of Atrogin-1 and MuRF1 gene expression alleviates muscle loss and improves the impaired myotube formation, indicating that oligonol supplementation may be useful for the attenuation of myotube loss.
Keywords: Atrogin-1 and MuRF1; NF-κB; diabetes; flavanol-rich lychee fruit extract; muscle loss.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Forkhead box O3 plays a role in skeletal muscle atrophy through expression of E3 ubiquitin ligases MuRF-1 and atrogin-1 in Cushing's syndrome.Am J Physiol Endocrinol Metab. 2017 Jun 1;312(6):E495-E507. doi: 10.1152/ajpendo.00389.2016. Epub 2017 Feb 28. Am J Physiol Endocrinol Metab. 2017. PMID: 28246104
-
Flavanol-rich lychee fruit extract alleviates diet-induced insulin resistance via suppressing mTOR/SREBP-1 mediated lipogenesis in liver and restoring insulin signaling in skeletal muscle.Mol Nutr Food Res. 2016 Oct;60(10):2288-2296. doi: 10.1002/mnfr.201501064. Epub 2016 Jul 5. Mol Nutr Food Res. 2016. PMID: 27161245
-
Oligonol Alleviates Sarcopenia by Regulation of Signaling Pathways Involved in Protein Turnover and Mitochondrial Quality.Mol Nutr Food Res. 2019 May;63(10):e1801102. doi: 10.1002/mnfr.201801102. Epub 2019 Feb 28. Mol Nutr Food Res. 2019. PMID: 30793867
-
Oligonol, a low-molecular-weight polyphenol derived from lychee fruit, attenuates gluco-lipotoxicity-mediated renal disorder in type 2 diabetic db/db mice.Drug Discov Ther. 2015 Feb;9(1):13-22. doi: 10.5582/ddt.2015.01003. Drug Discov Ther. 2015. PMID: 25788048 Review.
-
Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1.Am J Physiol Endocrinol Metab. 2014 Sep 15;307(6):E469-84. doi: 10.1152/ajpendo.00204.2014. Epub 2014 Aug 5. Am J Physiol Endocrinol Metab. 2014. PMID: 25096180 Free PMC article. Review.
Cited by
-
Effect of oligonol, a lychee-derived polyphenol, on skeletal muscle in ovariectomized rats by regulating body composition, protein turnover, and mitochondrial quality signaling.Food Sci Nutr. 2022 Mar 11;10(4):1184-1194. doi: 10.1002/fsn3.2750. eCollection 2022 Apr. Food Sci Nutr. 2022. PMID: 35432979 Free PMC article.
-
Pharmacological Properties of Polyphenols: Bioavailability, Mechanisms of Action, and Biological Effects in In Vitro Studies, Animal Models, and Humans.Biomedicines. 2021 Aug 23;9(8):1074. doi: 10.3390/biomedicines9081074. Biomedicines. 2021. PMID: 34440278 Free PMC article. Review.
-
Effects of tannase-converted green tea extract on skeletal muscle development.BMC Complement Med Ther. 2020 Feb 11;20(1):47. doi: 10.1186/s12906-020-2827-7. BMC Complement Med Ther. 2020. PMID: 32046706 Free PMC article.
-
Moderate Exercise Suppresses NF-κB Signaling and Activates the SIRT1-AMPK-PGC1α Axis to Attenuate Muscle Loss in Diabetic db/db Mice.Front Physiol. 2018 May 29;9:636. doi: 10.3389/fphys.2018.00636. eCollection 2018. Front Physiol. 2018. PMID: 29896118 Free PMC article.
-
Oligonol®, an Oligomerized Polyphenol from Litchi chinensis, Enhances Branched-Chain Amino Acid Transportation and Catabolism to Alleviate Sarcopenia.Int J Mol Sci. 2024 Oct 27;25(21):11549. doi: 10.3390/ijms252111549. Int J Mol Sci. 2024. PMID: 39519101 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

