SIMPLIFY-1: A Phase III Randomized Trial of Momelotinib Versus Ruxolitinib in Janus Kinase Inhibitor-Naïve Patients With Myelofibrosis
- PMID: 28930494
- PMCID: PMC6553796
- DOI: 10.1200/JCO.2017.73.4418
SIMPLIFY-1: A Phase III Randomized Trial of Momelotinib Versus Ruxolitinib in Janus Kinase Inhibitor-Naïve Patients With Myelofibrosis
Abstract
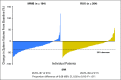
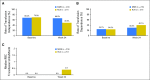
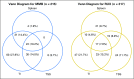
Purpose We evaluated the efficacy and safety of momelotinib, a potent and selective Janus kinase 1 and 2 inhibitor (JAKi), compared with ruxolitinib, in JAKi-naïve patients with myelofibrosis. Patients and Methods Patients (N = 432) with high risk or intermediate-2 risk or symptomatic intermediate-1 risk myelofibrosis were randomly assigned to receive 24 weeks of treatment with momelotinib 200 mg once daily or ruxolitinib 20 mg twice a day (or per label), after which all patients could receive open-label momelotinib. The primary end point was a ≥ 35% reduction in spleen volume at 24 weeks of therapy. Secondary end points were rates of symptom response and effects on RBC transfusion requirements. Results A ≥ 35% reduction in spleen volume at week 24 was achieved by a similar proportion of patients in both treatment arms: 26.5% of the momelotinib group and 29% of the ruxolitinib group (noninferior; P = .011). A ≥ 50% reduction in the total symptom score was observed in 28.4% and 42.2% of patients who received momelotinib and ruxolitinib, respectively, indicating that noninferiority was not met ( P = .98). Transfusion rate, transfusion independence, and transfusion dependence were improved with momelotinib (all with nominal P ≤ .019). The most common grade ≥ 3 hematologic abnormalities in either group were thrombocytopenia and anemia. Grade ≥ 3 infections occurred in 7% of patients who received momelotinib and 3% of patients who received ruxolitinib. Treatment-emergent peripheral neuropathy occurred in 10% of patients who received momelotinib (all grade ≤ 2) and 5% of patients who received ruxolitinib (all grade ≤ 3). Conclusion In JAKi-naïve patients with myelofibrosis, 24 weeks of momelotinib treatment was noninferior to ruxolitinib for spleen response but not for symptom response. Momelotinib treatment was associated with a reduced transfusion requirement.
Figures

References
-
- Levine RL, Pardanani A, Tefferi A, et al. : Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer 7:673-683, 2007 - PubMed
-
- Delhommeau F, Jeziorowska D, Marzac C, et al. : Molecular aspects of myeloproliferative neoplasms. Int J Hematol 91:165-173, 2010 - PubMed
-
- Klampfl T, Gisslinger H, Harutyunyan AS, et al. : Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 369:2379-2390, 2013 - PubMed
-
- Pardanani A: JAK2 inhibitor therapy in myeloproliferative disorders: Rationale, preclinical studies and ongoing clinical trials. Leukemia 22:23-30, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

