Evaluating the morphology of the left atrial appendage by a transesophageal echocardiographic 3-dimensional printed model
- PMID: 28930824
- PMCID: PMC5617691
- DOI: 10.1097/MD.0000000000007865
Evaluating the morphology of the left atrial appendage by a transesophageal echocardiographic 3-dimensional printed model
Abstract
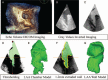
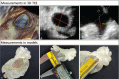
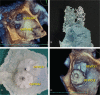
The novel 3-dimensional printing (3DP) technique has shown its ability to assist personalized cardiac intervention therapy. This study aimed to determine the feasibility of 3D-printed left atrial appendage (LAA) models based on 3D transesophageal echocardiography (3D TEE) data and their application value in treating LAA occlusions.Eighteen patients with transcatheter LAA occlusion, and preprocedure 3D TEE and cardiac computed tomography were enrolled. 3D TEE volumetric data of the LAA were acquired and postprocessed for 3DP. Two types of 3D models of the LAA (ie, hard chamber model and flexible wall model) were printed by a 3D printer. The morphological classification and lobe identification of the LAA were assessed by the 3D chamber model, and LAA dimensions were measured via the 3D wall model. Additionally, a simulation operative rehearsal was performed on the 3D models in cases of challenging LAA morphology for the purpose of understanding the interactions between the device and the model.Three-dimensional TEE volumetric data of the LAA were successfully reprocessed and printed as 3D LAA chamber models and 3D LAA wall models in all patients. The consistency of the morphological classifications of the LAA based on 3D models and cardiac computed tomography was 0.92 (P < .01). The differences between the LAA ostium dimensions and depth measured using the 3D models were not significant from those measured on 3D TEE (P > .05). A simulation occlusion was successfully performed on the 3D model of the 2 challenging cases and compared with the real procedure.The echocardiographic 3DP technique is feasible and accurate in reflecting the spatial morphology of the LAA, which may be promising for the personalized planning of transcatheter LAA occlusion.
Conflict of interest statement
The authors report no conflicts of interest.
Figures


Similar articles
-
The value of the left atrial appendage orifice perimeter of 3D model based on 3D TEE data in the choice of device size of LAmbre™ occluder.Int J Cardiovasc Imaging. 2019 Oct;35(10):1841-1851. doi: 10.1007/s10554-019-01627-4. Epub 2019 May 27. Int J Cardiovasc Imaging. 2019. PMID: 31134413
-
Roles of real-time three-dimensional transesophageal echocardiography in peri-operation of transcatheter left atrial appendage closure.Medicine (Baltimore). 2017 Jan;96(4):e5637. doi: 10.1097/MD.0000000000005637. Medicine (Baltimore). 2017. PMID: 28121919 Free PMC article.
-
The Value of 3D Printing Models of Left Atrial Appendage Using Real-Time 3D Transesophageal Echocardiographic Data in Left Atrial Appendage Occlusion: Applications toward an Era of Truly Personalized Medicine.Cardiology. 2016;135(4):255-261. doi: 10.1159/000447444. Epub 2016 Aug 19. Cardiology. 2016. PMID: 27537503
-
A systematic review of the use of 3D printing in left atrial appendage occlusion procedures.J Cardiovasc Electrophysiol. 2022 Nov;33(11):2367-2374. doi: 10.1111/jce.15658. Epub 2022 Sep 7. J Cardiovasc Electrophysiol. 2022. PMID: 35989544
-
Role of cardiac imaging and three-dimensional printing in percutaneous appendage closure.Arch Cardiovasc Dis. 2018 Jun-Jul;111(6-7):411-420. doi: 10.1016/j.acvd.2018.04.005. Epub 2018 Jun 7. Arch Cardiovasc Dis. 2018. PMID: 29886007 Review.
Cited by
-
Three-dimensional printing in structural heart disease and intervention.Ann Transl Med. 2019 Oct;7(20):579. doi: 10.21037/atm.2019.09.73. Ann Transl Med. 2019. PMID: 31807560 Free PMC article. Review.
-
Preprocedural Planning of Left Atrial Appendage Occlusion: A Review of the Use of Additive Manufacturing.3D Print Addit Manuf. 2024 Feb 1;11(1):333-346. doi: 10.1089/3dp.2022.0373. Epub 2024 Feb 15. 3D Print Addit Manuf. 2024. PMID: 38389681 Free PMC article. Review.
-
Translating Imaging Into 3D Printed Cardiovascular Phantoms: A Systematic Review of Applications, Technologies, and Validation.JACC Basic Transl Sci. 2022 Apr 6;7(10):1050-1062. doi: 10.1016/j.jacbts.2022.01.002. eCollection 2022 Oct. JACC Basic Transl Sci. 2022. PMID: 36337920 Free PMC article. Review.
-
3D printing from transesophageal echocardiography for planning mitral paravalvular leak closure - feasibility study.Postepy Kardiol Interwencyjnej. 2023 Sep;19(3):270-276. doi: 10.5114/aic.2023.131481. Epub 2023 Sep 27. Postepy Kardiol Interwencyjnej. 2023. PMID: 37854960 Free PMC article.
-
Clinical situations for which 3D printing is considered an appropriate representation or extension of data contained in a medical imaging examination: adult cardiac conditions.3D Print Med. 2020 Sep 23;6(1):24. doi: 10.1186/s41205-020-00078-1. 3D Print Med. 2020. PMID: 32965536 Free PMC article.
References
-
- Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–429. - PubMed
-
- Huisman MV, Rothman KJ, Paquette M, et al. The changing landscape for stroke prevention in AF: findings from the GLORIA-AF registry phase 2. J Am Coll Cardiol 2017;69:777–85. - PubMed
-
- Deedwania P, Acharya T. Anticoagulation in atrial fibrillation: is the paradigm really shifting? J Am Coll Cardiol 2017;69:786–8. - PubMed
-
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–78. - PubMed
-
- Chanda A, Reilly JP. Left atrial appendage occlusion for stroke prevention. Prog Cardiovasc Dis 2017;59:626–35. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

