An Enrichment Strategy Yields Seven Novel Single Nucleotide Polymorphisms Associated With Mortality and Altered Th17 Responses Following Blunt Trauma
- PMID: 28930911
- PMCID: PMC5809248
- DOI: 10.1097/SHK.0000000000000987
An Enrichment Strategy Yields Seven Novel Single Nucleotide Polymorphisms Associated With Mortality and Altered Th17 Responses Following Blunt Trauma
Abstract
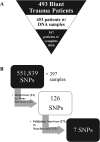
Trauma is the leading cause of death worldwide for individuals under the age of 55. Interpatient genomic differences, in the form of candidate single-nucleotide polymorphisms (SNPs), have been associated previously with adverse outcomes after trauma. However, the utility of these SNPs to predict outcomes based on a meaningful endpoint such as survival is as yet undefined. We hypothesized that specific SNP haplotypes could segregate trauma survivors from non-survivors. Genomic DNA samples were obtained from 453 blunt trauma patients, for whom complete daily clinical and biomarker data were available for 397. Of these, 13 patients were non-survivors and the remaining 384 were survivors. All 397 DNA samples were amplified, fragmented, and examined for 551,839 SNPs using the Illumina Infinium CoreExome-24 v1.1 BeadChip (Illumina). To enrich for likely important SNPs, we initially compared SNPs of the 13 non-survivors versus 13 matched survivors, who were matched algorithmically for injury severity score (ISS), age, and gender ratio. This initial enrichment yielded 126 SNPs; a further comparison to the haplotypes of the remaining 371 survivors yielded a final total of 7 SNPs that distinguished survivors from non-survivors. Furthermore, severely injured survivors with the same seven SNPs as non-survivor exhibited distinct inflammatory responses from similarly injured survivors without those SNPs, and specifically had evidence of altered Th17 cell phenotypes based on computational modeling. These studies suggest an interaction among genetic polymorphism, injury severity, and initial inflammatory responses in driving trauma outcomes.
Figures



References
-
- Namas RA, Mi Q, Namas R, Almahmoud K, Zaaqoq AM, Abdul-Malak O, Azhar N, Day J, Abboud A, Zamora R, et al. Insights into the role of chemokines, damage-associated molecular patterns, and lymphocyte-derived mediators from computational models of trauma-induced inflammation. Antiox. Redox Signaling. 2015;10:1370–1387. - PMC - PubMed
-
- Hildebrand F, Mommsen P, Frink M, van Griensven M, Krettek C. Genetic predisposition for development of complications in multiple trauma patients. Shock. 2011;35(5):440–8. - PubMed
-
- Syvanen AC. Accessing genetic variation: genotyping single nucleotide polymorphisms. Nat Rev Genet. 2001;2(12):930–42. - PubMed
-
- Bronkhorst MW, P Patka P, van Lieshout EM. Effects of sequence variations in innate immune response genes on infectious outcome in trauma patients: A comprehensive review. Shock. 2015;44(5):390–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

