The Protective Effect of A Short Peptide Derived From Cold-Inducible RNA-Binding Protein in Renal Ischemia-Reperfusion Injury
- PMID: 28930914
- PMCID: PMC5809308
- DOI: 10.1097/SHK.0000000000000988
The Protective Effect of A Short Peptide Derived From Cold-Inducible RNA-Binding Protein in Renal Ischemia-Reperfusion Injury
Abstract
Extracellular cold-inducible RNA-binding protein (CIRP) functions as damage-associated molecular pattern and has been demonstrated to be responsible in part for the damage occurring after renal ischemia-reperfusion (I/R). A short peptide derived from CIRP, named C23, binds to myeloid differentiation factor 2, a Toll-like receptor 4 coreceptor. We hypothesize that C23 reduces renal ischemia-reperfusion (RIR) injury by blocking CIRP. We observed that pretreatment with C23 significantly decreased the levels of recombinant mouse CIRP-induced tumor necrosis factor-α (TNF-α) in a dose-dependent fashion in cultured macrophages. C57BL/6 mice were subjected to bilateral renal pedicle clamps for 35 min to induce ischemia, followed by reperfusion for 24 h and harvest of blood and renal tissue. C23 peptide (8 mg/kg) or vehicle was injected intraperitoneally at the beginning of reperfusion. Plasma TNF-α, interleukin 1 beta (IL-1β), and IL-6 levels were decreased in C23-treated RIR mice as compared with vehicle-treated mice by 74%, 85%, and 68%, respectively. Expressions of TNF-α and keratinocyte chemoattractant in the kidneys from C23-treated mice were decreased by 55% and 60%, respectively. Expression of kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin in the kidney of C23-treated mice were significantly reduced by 46% and 55%, respectively. Renal tissue histological assessments revealed significant reduction in damage score by 44% in C23-treated mice. Finally, a survival study revealed a significant survival advantage with a 70% survival rate in C23 group vs. 37% in vehicle group. Thus, C23 has potential as a novel therapy for the patients suffering from I/R-induced renal injury.
Conflict of interest statement
One of the authors (PW) is an inventor of the pending PCT application # WO2015048083 A1: “Peptides inhibiting cold-inducible rna binding protein activity”. TheraSource has an option to license this technology. Other authors report no financial conflicts of interest.
Figures

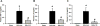
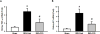




Similar articles
-
Cold-inducible RNA-binding protein-derived peptide C23 attenuates inflammation and tissue injury in a murine model of intestinal ischemia-reperfusion.Surgery. 2018 Dec;164(6):1191-1197. doi: 10.1016/j.surg.2018.06.048. Epub 2018 Aug 25. Surgery. 2018. PMID: 30154017 Free PMC article.
-
An eCIRP inhibitor attenuates fibrosis and ferroptosis in ischemia and reperfusion induced chronic kidney disease.Mol Med. 2025 Jan 10;31(1):11. doi: 10.1186/s10020-025-01071-2. Mol Med. 2025. PMID: 39794717 Free PMC article.
-
A cold-inducible RNA-binding protein (CIRP)-derived peptide attenuates inflammation and organ injury in septic mice.Sci Rep. 2018 Feb 12;8(1):3052. doi: 10.1038/s41598-017-13139-z. Sci Rep. 2018. PMID: 29434211 Free PMC article.
-
Exosome-derived CIRP: An amplifier of inflammatory diseases.Front Immunol. 2023 Feb 14;14:1066721. doi: 10.3389/fimmu.2023.1066721. eCollection 2023. Front Immunol. 2023. PMID: 36865547 Free PMC article. Review.
-
Cold-inducible RNA-binding protein (CIRP) in inflammatory diseases: Molecular insights of its associated signalling pathways.Scand J Immunol. 2021 Jan;93(1):e12949. doi: 10.1111/sji.12949. Epub 2020 Sep 8. Scand J Immunol. 2021. PMID: 32738154 Review.
Cited by
-
Cold-inducible RNA-binding protein-derived peptide C23 attenuates inflammation and tissue injury in a murine model of intestinal ischemia-reperfusion.Surgery. 2018 Dec;164(6):1191-1197. doi: 10.1016/j.surg.2018.06.048. Epub 2018 Aug 25. Surgery. 2018. PMID: 30154017 Free PMC article.
-
Damage-Associated Molecular Patterns As Double-Edged Swords in Sepsis.Antioxid Redox Signal. 2021 Nov 20;35(15):1308-1323. doi: 10.1089/ars.2021.0008. Epub 2021 Mar 30. Antioxid Redox Signal. 2021. PMID: 33587003 Free PMC article. Review.
-
CIRBP promotes ferroptosis by interacting with ELAVL1 and activating ferritinophagy during renal ischaemia-reperfusion injury.J Cell Mol Med. 2021 Jun 10;25(13):6203-16. doi: 10.1111/jcmm.16567. Online ahead of print. J Cell Mol Med. 2021. PMID: 34114349 Free PMC article.
-
Necroptosis-Mediated eCIRP Release in Sepsis.J Inflamm Res. 2022 Jul 17;15:4047-4059. doi: 10.2147/JIR.S370615. eCollection 2022. J Inflamm Res. 2022. PMID: 35873387 Free PMC article.
-
CIRP contributes to multiple organ damage in acute pancreatitis by increasing endothelial permeability.Commun Biol. 2025 Mar 10;8(1):403. doi: 10.1038/s42003-025-07772-y. Commun Biol. 2025. PMID: 40065057 Free PMC article.
References
-
- Kanbay M, Kasapoglu B, Perazella MA. Acute tubular necrosis and pre-renal acute kidney injury: utility of urine microscopy in their evaluation- a systematic review. Int Urol Nephrol. 2010;42(2):425–433. - PubMed
-
- Liangos O, Wald R, O’Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1(1):43–51. - PubMed
-
- Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7(4):189–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

