Abnormalities in substance P neurokinin-1 receptor binding in key brainstem nuclei in sudden infant death syndrome related to prematurity and sex
- PMID: 28931039
- PMCID: PMC5607183
- DOI: 10.1371/journal.pone.0184958
Abnormalities in substance P neurokinin-1 receptor binding in key brainstem nuclei in sudden infant death syndrome related to prematurity and sex
Abstract
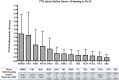
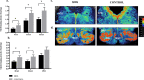
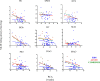
Sudden infant death syndrome (SIDS) involves failure of arousal to potentially life threatening events, including hypoxia, during sleep. While neuronal dysfunction and abnormalities in neurotransmitter systems within the medulla oblongata have been implicated, the specific pathways associated with autonomic and cardiorespiratory failure are unknown. The neuropeptide substance P (SP) and its tachykinin neurokinin-1 receptor (NK1R) have been shown to play an integral role in the modulation of homeostatic function in the medulla, including regulation of respiratory rhythm generation, integration of cardiovascular control, and modulation of the baroreceptor reflex and mediation of the chemoreceptor reflex in response to hypoxia. Abnormalities in SP neurotransmission may therefore result in autonomic dysfunction during sleep and contribute to SIDS deaths. [125I] Bolton Hunter SP autoradiography was used to map the distribution and density of the SP, NK1R to 13 specific nuclei intimately related to cardiorespiratory function and autonomic control in the human infant medulla of 55 SIDS and 21 control (non-SIDS) infants. Compared to controls, SIDS cases exhibited a differential, abnormal developmental profile of the SP/NK1R system in the medulla. Furthermore the study revealed significantly decreased NK1R binding within key medullary nuclei in SIDS cases, principally in the nucleus tractus solitarii (NTS) and all three subdivisions of the inferior portion of the olivo-cerebellar complex; the principal inferior olivary complex (PIO), medial accessory olive (MAO) and dorsal accessory olive (DAO). Altered NK1R binding was significantly influenced by prematurity and male sex, which may explain the increased risk of SIDS in premature and male infants. Abnormal NK1R binding in these medullary nuclei may contribute to the defective interaction of critical medullary mechanisms with cerebellar sites, resulting in an inability of a SIDS infant to illicit appropriate respiratory and motor responses to life threatening challenges during sleep. These observations support the concept that abnormalities in a multi-neurotransmitter network within key nuclei of the medullary homeostatic system may underlie the pathogenesis of a subset of SIDS cases.
Conflict of interest statement
Figures


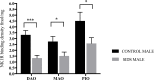
Similar articles
-
The potential role of substance P in brainstem homeostatic control in the pathogenesis of sudden infant death syndrome (SIDS).Neuropeptides. 2018 Aug;70:1-8. doi: 10.1016/j.npep.2018.02.006. Epub 2018 May 5. Neuropeptides. 2018. PMID: 29908886 Review.
-
Normative distribution of substance P and its tachykinin neurokinin-1 receptor in the medullary serotonergic network of the human infant during postnatal development.Brain Res Bull. 2018 Mar;137:319-328. doi: 10.1016/j.brainresbull.2018.01.009. Epub 2018 Jan 11. Brain Res Bull. 2018. PMID: 29331576
-
Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome.J Neuropathol Exp Neurol. 2000 May;59(5):377-84. doi: 10.1093/jnen/59.5.377. J Neuropathol Exp Neurol. 2000. PMID: 10888367
-
Multiple serotonergic brainstem abnormalities in sudden infant death syndrome.JAMA. 2006 Nov 1;296(17):2124-32. doi: 10.1001/jama.296.17.2124. JAMA. 2006. PMID: 17077377
-
Brainstem mechanisms underlying the sudden infant death syndrome: evidence from human pathologic studies.Dev Psychobiol. 2009 Apr;51(3):223-33. doi: 10.1002/dev.20367. Dev Psychobiol. 2009. PMID: 19235901 Review.
Cited by
-
Substance P Concentration in Gestational Diabetes and Excessive Gestational Weight Gain and Its Impact on Neonatal Anthropometry.Int J Mol Sci. 2024 Mar 28;25(7):3759. doi: 10.3390/ijms25073759. Int J Mol Sci. 2024. PMID: 38612572 Free PMC article.
-
Genetic variants in eleven central and peripheral chemoreceptor genes in sudden infant death syndrome.Pediatr Res. 2022 Oct;92(4):1026-1033. doi: 10.1038/s41390-021-01899-4. Epub 2022 Feb 1. Pediatr Res. 2022. PMID: 35102300 Free PMC article.
-
Cardiovascular autonomic dysfunction in sudden infant death syndrome.Clin Auton Res. 2018 Dec;28(6):535-543. doi: 10.1007/s10286-017-0490-y. Epub 2018 Jan 3. Clin Auton Res. 2018. PMID: 29299712 Review.
-
Impaired motor control in SIDS infants.Int J Legal Med. 2018 Sep;132(5):1389. doi: 10.1007/s00414-018-1788-6. Epub 2018 Jan 29. Int J Legal Med. 2018. PMID: 29380123 No abstract available.
-
Why is a prone sleeping position dangerous for certain infants?Forensic Sci Med Pathol. 2018 Mar;14(1):114-116. doi: 10.1007/s12024-017-9941-y. Epub 2017 Dec 14. Forensic Sci Med Pathol. 2018. PMID: 29243157
References
-
- Kinney HC, Thach BT. The sudden infant death syndrome. The New England journal of medicine. 2009;361(8):795–805. Epub 2009/08/21. doi: 10.1056/NEJMra0803836 ; PubMed Central PMCID: PMCPMC3268262. - DOI - PMC - PubMed
-
- Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biology of the neonate. 1994;65(3–4):194–7. Epub 1994/01/01. . - PubMed
-
- Takashima S, Becker LE. Developmental abnormalities of medullary "respiratory centers" in sudden infant death syndrome. Experimental neurology. 1985;90(3):580–7. Epub 1985/12/01. . - PubMed
-
- Sridhar R, Thach BT, Kelly DH, Henslee JA. Characterization of successful and failed autoresuscitation in human infants, including those dying of SIDS. Pediatric pulmonology. 2003;36(2):113–22. Epub 2003/07/02. doi: 10.1002/ppul.10287 . - DOI - PubMed
-
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, et al. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA: the journal of the American Medical Association. 2006;296(17):2124–32. Epub 2006/11/02. doi: 10.1001/jama.296.17.2124 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

