Effect of extracytoplasmic function sigma factors on autoaggregation, hemagglutination, and cell surface properties of Porphyromonas gingivalis
- PMID: 28931045
- PMCID: PMC5607195
- DOI: 10.1371/journal.pone.0185027
Effect of extracytoplasmic function sigma factors on autoaggregation, hemagglutination, and cell surface properties of Porphyromonas gingivalis
Abstract
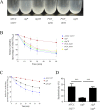
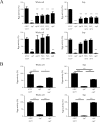
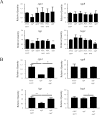
Porphyromonas gingivalis is a bacterium frequently isolated from chronic periodontal lesions and is involved in the development of chronic periodontitis. To colonize the gingival crevice, P. gingivalis has to adapt to environmental stresses. Microbial gene expression is regulated by transcription factors such as those in two-component systems and extracytoplasmic function (ECF) sigma factors. ECF sigma factors are involved in the regulation of environmental stress response genes; however, the roles of individual ECF sigma factors are largely unknown. The purpose of this study was to investigate the functions, including autoaggregation, hemagglutination, gingipain activity, susceptibility to antimicrobial agents, and surface structure formation, of P. gingivalis ECF sigma factors encoded by SigP (PGN_0274), SigCH (PGN_0319), PGN_0450, PGN_0970, and SigH (PGN_1740). Various physiological aspects of the sigP mutant were affected; autoaggregation was significantly decreased at 60 min (p < 0.001), hemagglutination activity was markedly reduced, and enzymatic activities of Kgp and Rgps were significantly decreased (p < 0.001). The other mutants also showed approximately 50% reduction in Rgps activity. Kgp activity was significantly reduced in the sigH mutant (p < 0.001). No significant differences in susceptibilities to tetracycline and ofloxacin were observed in the mutants compared to those of the wild-type strain. However, the sigP mutant displayed an increased susceptibility to ampicillin, whereas the PGN_0450 and sigH mutants showed reduced susceptibility. Transmission electron microscopy images revealed increased levels of outer membrane vesicles formed at the cell surfaces of the sigP mutant. These results indicate that SigP is important for bacterial surface-associated activities, including gingipain activity, autoaggregation, hemagglutination, vesicle formation, and antimicrobial susceptibility.
Conflict of interest statement
Figures




References
-
- Eisenstark A, Calcutt MJ, Becker-Hapak M, Ivanova A. Role of Escherichia coli rpoS and associated genes in defense against oxidative damage. Free Radic Biol Med 1996;21: 975–993. - PubMed
-
- Hengge-Aronis R. Signal transduction and regulatory mechanisms involved in control of the σs (RpoS) subunit of RNA polymerase. Microbiol Mol Biol Rev 2002;66: 373–395. doi: 10.1128/MMBR.66.3.373-395.2002 - DOI - PMC - PubMed
-
- Mascher T. Signaling diversity and evolution of extracytoplasmic function (ECF) sigma factors. Curr Opin Microbiol 2013;16: 148–155. doi: 10.1016/j.mib.2013.02.001 - DOI - PubMed
-
- Staron A, Sofia HJ, Dietrich S, Ulrich LE, Liesegang H, Mascher T. The third pillar of bacterial signal transduction: classification of the extracytoplasmic function (ECF) sigma factor protein family. Mol Microbiol 2009;74: 557–581. doi: 10.1111/j.1365-2958.2009.06870.x - DOI - PubMed
-
- Brooks BE, Buchanan SK. Signaling mechanisms for activation of extracytoplasmic function (ECF) sigma factors. Biochim Biophys Acta 2008;1778: 1930–1945. doi: 10.1016/j.bbamem.2007.06.005 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

