miR-181a decelerates proliferation in cutaneous squamous cell carcinoma by targeting the proto-oncogene KRAS
- PMID: 28931048
- PMCID: PMC5607211
- DOI: 10.1371/journal.pone.0185028
miR-181a decelerates proliferation in cutaneous squamous cell carcinoma by targeting the proto-oncogene KRAS
Abstract
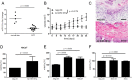
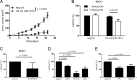
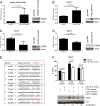
Cutaneous squamous cell carcinoma (SCC) is the second most common human skin cancer with a rapidly increasing incidence among the Caucasian population. Among the many regulators, responsible for cancer progression and growth, microRNAs (miRNA) are generally accepted as key players by now. In our current study we found that microRNA-181a (miR-181a) shows low abundance in SCC compared to normal epidermal skin. In vitro, miRNA downregulation in normal primary keratinocytes induced increased proliferation, while in vivo miR-181a downregulation in HaCaT normal keratinocytes showed tumor-like growth increase up to 50%. Inversely, upregulation of these miRNAs in cancer cells lead to reduced cellular proliferation and induction of apoptosis in vitro. An in vivo therapeutic model with induced miR-181a expression in SCC13 cancer cells reduced tumor formation in mice by 80%. Modulation of miR-181a levels showed an inverse correlation with the proto-oncogene KRAS both on mRNA and protein level by direct interaction. Knockdown of KRAS mimicked the anti-proliferative effects of miR-181a overexpression in patient-derived SCC cells and abolished the enhanced viability of HaCaT cells following miR-181a knockdown. Furthermore, phospho-ERK levels correlated with KRAS levels, suggesting that the observed effects were mediated via the MAPK signaling pathway. miR-181a seemed regulated during keratinocyte differentiation probably in order to amplify the tumor suppressive character of differentiation. Taken together, miR-181a plays a crucial tumor suppressive role in SCC by targeting KRAS and could be a promising candidate for a miRNA based therapy.
Conflict of interest statement
Figures

References
-
- Hofbauer GF, Bouwes Bavinck JN, Euvrard S. Organ transplantation and skin cancer: basic problems and new perspectives. Experimental dermatology. 2010;19(6):473–82. Epub 2010/05/21. doi: 10.1111/j.1600-0625.2010.01086.x . - DOI - PubMed
-
- Lohmann CM, Solomon AR. Clinicopathologic variants of cutaneous squamous cell carcinoma. Advances in anatomic pathology. 2001;8(1):27–36. Epub 2001/01/11. . - PubMed
-
- Ratushny V, Gober MD, Hick R, Ridky TW, Seykora JT. From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma. The Journal of clinical investigation. 2012;122(2):464–72. Epub 2012/02/02. doi: 10.1172/JCI57415 ; PubMed Central PMCID: PMCPMC3266779. - DOI - PMC - PubMed
-
- Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(22):10124–8. Epub 1991/11/15. ; PubMed Central PMCID: PMCPMC52880. - PMC - PubMed
-
- Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. The New England journal of medicine. 2003;348(17):1681–91. Epub 2003/04/25. doi: 10.1056/NEJMra022137 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

