The C5a/C5aR1 axis controls the development of experimental allergic asthma independent of LysM-expressing pulmonary immune cells
- PMID: 28931049
- PMCID: PMC5607179
- DOI: 10.1371/journal.pone.0184956
The C5a/C5aR1 axis controls the development of experimental allergic asthma independent of LysM-expressing pulmonary immune cells
Abstract
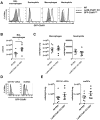
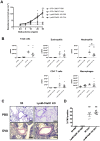
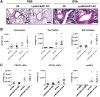
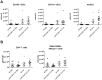
C5a regulates the development of maladaptive immune responses in allergic asthma mainly through the activation of C5a receptor 1 (C5aR1). Yet, the cell types and the mechanisms underlying this regulation are ill-defined. Recently, we described increased C5aR1 expression in lung tissue eosinophils but decreased expression in airway and pulmonary macrophages as well as in pulmonary CD11b+ conventional dendritic cells (cDCs) and monocyte-derived DCs (moDCs) during the allergic effector phase using a floxed green fluorescent protein (GFP)-C5aR1 knock-in mouse. Here, we determined the role of C5aR1 signaling in neutrophils, moDCs and macrophages for the pulmonary recruitment of such cells and the importance of C5aR1-mediated activation of LysM-expressing cells for the development of allergic asthma. We used LysM-C5aR1 KO mice with a specific deletion of C5aR1 in LysMCre-expressing cells and confirmed the specific deletion of C5aR1 in neutrophils, macrophages and moDCs in the airways and/or the lung tissue. We found that alveolar macrophage numbers were significantly increased in LysM-C5aR1 KO mice. Induction of ovalbumin (OVA)-driven experimental allergic asthma in GFP-C5aR1fl/fl and LysM-C5aR1 KO mice resulted in strong but similar airway resistance, mucus production and Th2/Th17 cytokine production. In contrast, the number of airway but not of pulmonary neutrophils was lower in LysM-C5aR1 KO as compared with GFP-C5aR1fl/fl mice. The recruitment of macrophages, cDCs, moDCs, T cells and type 2 innate lymphoid cells was not altered in LysM-C5aR1 KO mice. Our findings demonstrate that C5aR1 is critical for steady state control of alveolar macrophage numbers and the transition of neutrophils from the lung into the airways in OVA-driven allergic asthma. However, C5aR1 activation of LysM-expressing cells plays a surprisingly minor role in the recruitment and activation of such cells and the development of the allergic phenotype in OVA-driven experimental allergic asthma.
Conflict of interest statement
Figures

References
-
- Laumonnier Y, Wiese AV, Figge J, Karsten C. Regulation and function of anaphylatoxins and their receptors in allergic asthma. Mol Immunol. 2017;84:51–6. Epub 2016/12/06. doi: 10.1016/j.molimm.2016.11.013 . - DOI - PubMed
-
- Kohl J, Baelder R, Lewkowich IP, Pandey MK, Hawlisch H, Wang L, et al. A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J Clin Invest. 2006;116(3):783–96. Epub 2006/03/03. doi: 10.1172/JCI26582 - DOI - PMC - PubMed
-
- Staab EB, Sanderson SD, Wells SM, Poole JA. Treatment with the C5a receptor/CD88 antagonist PMX205 reduces inflammation in a murine model of allergic asthma. Int Immunopharmacol. 2014;21(2):293–300. Epub 2014/05/27. doi: 10.1016/j.intimp.2014.05.008 - DOI - PMC - PubMed
-
- Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, et al. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11(10):928–35. Epub 2010/08/31. doi: 10.1038/ni.1926 - DOI - PMC - PubMed
-
- Weaver DJ Jr., Reis ES, Pandey MK, Kohl G, Harris N, Gerard C, et al. C5a receptor-deficient dendritic cells promote induction of Treg and Th17 cells. Eur J Immunol. 2010;40(3):710–21. Epub 2009/12/18. doi: 10.1002/eji.200939333 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

