Identification and molecular characterization of a metagenome-derived L-lysine decarboxylase gene from subtropical soil microorganisms
- PMID: 28931053
- PMCID: PMC5607190
- DOI: 10.1371/journal.pone.0185060
Identification and molecular characterization of a metagenome-derived L-lysine decarboxylase gene from subtropical soil microorganisms
Abstract
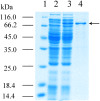
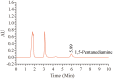
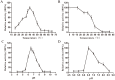
L-lysine decarboxylase (LDC, EC 4.1.1.18) is a key enzyme in the decarboxylation of L-lysine to 1,5-pentanediamine and efficiently contributes significance to biosynthetic capability. Metagenomic technology is a shortcut approach used to obtain new genes from uncultured microorganisms. In this study, a subtropical soil metagenomic library was constructed, and a putative LDC gene named ldc1E was isolated by function-based screening strategy through the indication of pH change by L-lysine decarboxylation. Amino acid sequence comparison and homology modeling indicated the close relation between Ldc1E and other putative LDCs. Multiple sequence alignment analysis revealed that Ldc1E contained a highly conserved motif Ser-X-His-Lys (Pxl), and molecular docking results showed that this motif was located in the active site and could combine with the cofactor pyridoxal 5'-phosphate. The ldc1E gene was subcloned into the pET-30a(+) vector and highly expressed in Escherichia coli BL21 (DE3) pLysS. The recombinant protein was purified to homogeneity. The maximum activity of Ldc1E occurred at pH 6.5 and 40°C using L-lysine monohydrochloride as the substrate. Recombinant Ldc1E had apparent Km, kcat, and kcat/Km values of 1.08±0.16 mM, 5.09±0.63 s-1, and 4.73×103 s-1 M-1, respectively. The specific activity of Ldc1E was 1.53±0.06 U mg-1 protein. Identifying a metagenome-derived LDC gene provided a rational reference for further gene modifications in industrial applications.
Conflict of interest statement
Figures


Similar articles
-
Molecular cloning and functional characterization of a novel decarboxylase from uncultured microorganisms.Biochem Biophys Res Commun. 2007 Jun 1;357(2):421-6. doi: 10.1016/j.bbrc.2007.03.159. Epub 2007 Apr 3. Biochem Biophys Res Commun. 2007. PMID: 17420004
-
A novel D-amino acid oxidase from a contaminated agricultural soil metagenome and its characterization.Antonie Van Leeuwenhoek. 2015 Jun;107(6):1615-23. doi: 10.1007/s10482-015-0457-8. Epub 2015 Apr 22. Antonie Van Leeuwenhoek. 2015. PMID: 25900453
-
Identification of a metagenome-derived prephenate dehydrogenase gene from an alkaline-polluted soil microorganism.Antonie Van Leeuwenhoek. 2013 Jun;103(6):1209-19. doi: 10.1007/s10482-013-9899-z. Epub 2013 Mar 12. Antonie Van Leeuwenhoek. 2013. PMID: 23479063
-
[Molecular engineering and immobilization of lysine decarboxylase for synthesis of 1, 5-diaminopentane: a review].Sheng Wu Gong Cheng Xue Bao. 2022 Dec 25;38(12):4403-4419. doi: 10.13345/j.cjb.220400. Sheng Wu Gong Cheng Xue Bao. 2022. PMID: 36593185 Review. Chinese.
-
The Cytokinin-Activating LOG-Family Proteins Are Not Lysine Decarboxylases.Trends Biochem Sci. 2018 Apr;43(4):232-236. doi: 10.1016/j.tibs.2018.01.002. Epub 2018 Mar 7. Trends Biochem Sci. 2018. PMID: 29525484 Review.
Cited by
-
Structural and functional analysis of the Francisella lysine decarboxylase as a key actor in oxidative stress resistance.Sci Rep. 2021 Jan 13;11(1):972. doi: 10.1038/s41598-020-79611-5. Sci Rep. 2021. PMID: 33441661 Free PMC article.
-
Prospecting Microbial Genomes for Biomolecules and Their Applications.Indian J Microbiol. 2022 Dec;62(4):516-523. doi: 10.1007/s12088-022-01040-x. Epub 2022 Sep 12. Indian J Microbiol. 2022. PMID: 36458216 Free PMC article. Review.
-
Green chemical and biological synthesis of cadaverine: recent development and challenges.RSC Adv. 2021 Jul 7;11(39):23922-23942. doi: 10.1039/d1ra02764f. eCollection 2021 Jul 6. RSC Adv. 2021. PMID: 35479032 Free PMC article. Review.
-
Molecular Characterization and Directed Evolution of a Metagenome-Derived l-Cysteine Sulfinate Decarboxylase.Food Technol Biotechnol. 2018 Mar;56(1):117-123. doi: 10.17113/ftb.56.01.18.5415. Food Technol Biotechnol. 2018. PMID: 29796005 Free PMC article.
-
Effective Biodegradation of Aflatoxin B1 Using the Bacillus licheniformis (BL010) Strain.Toxins (Basel). 2018 Nov 26;10(12):497. doi: 10.3390/toxins10120497. Toxins (Basel). 2018. PMID: 30486278 Free PMC article.
References
-
- Bunsupa S, Katayama K, Ikeura E, Oikawa A, Toyooka K, Saito K, et al. Lysine decarboxylase catalyzes the first step of quinolizidine alkaloid biosynthesis and coevolved with alkaloid production in leguminosae. Plant Cell. 2012;24(3): 1202–1216. doi: 10.1105/tpc.112.095885 - DOI - PMC - PubMed
-
- Bhatia SK, Bhatia RK, Yang YH. Biosynthesis of polyesters and polyamide building blocks using microbial fermentation and biotransformation. Rev Environ Sci Biotechnol. 2016;15(4): 639–663. doi: 10.1007/s11157-016-9415-9 - DOI
-
- Kind S, Kreye S, Wittmann C. Metabolic engineering of cellular transport for overproduction of the platform chemical 1,5-diaminopentane in Corynebacterium glutamicum. Metab Eng. 2011;13(5): 617–627. doi: 10.1016/j.ymben.2011.07.006 - DOI - PubMed
-
- Kind S, Neubauer S, Becker J, Yamamoto M, Völkert M, Abendroth Gv, et al. From zero to hero-production of bio-based nylon from renewable resources using engineered Corynebacterium glutamicum. Metab Eng. 2014;25: 113–123. doi: 10.1016/j.ymben.2014.05.007 - DOI - PubMed
-
- Tsuge Y, Kawaguchi H, Sasaki K, Kondo A. Engineering cell factories for producing building block chemicals for bio-polymer synthesis. Microb Cell Fact. 2016;15: 19 doi: 10.1186/s12934-016-0411-0 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

