Factors associated with hepatitis C prevalence differ by the stage of liver fibrosis: A cross-sectional study in the general population in Poland, 2012-2016
- PMID: 28931062
- PMCID: PMC5607182
- DOI: 10.1371/journal.pone.0185055
Factors associated with hepatitis C prevalence differ by the stage of liver fibrosis: A cross-sectional study in the general population in Poland, 2012-2016
Abstract
Background & aims: There is a considerable burden of hepatitis C in Europe related to the lack of prompt diagnosis. We aimed to estimate the prevalence and related risk factors of HCV infections by the stages of liver fibrosis, using non-invasive methods, to understand testing needs in Poland.
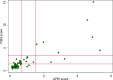
Methods: A cross-sectional study was conducted in 2012-2016 adopting a stratified random sampling of primary health care units followed by systematic sampling of patients within each unit. Study participants filled a questionnaire and donated blood for laboratory HCV testing. Additionally, the results of liver function tests and platelet count were collected to calculate APRI and FIB-4 scores. Cases were classified according to the level of fibrosis: 'significant fibrosis' (APRI≥0.7 or FIB4≥1.45) and 'no significant fibrosis' (APRI<0.7 and FIB4<1.45).
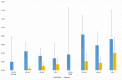
Results: Of 21 875 study participants, 102 were HCV-RNA positive. Prevalence of HCV infections and significant fibrosis was estimated at 0.47% (95% CI 0.38% - 0.57%) and 0.12% (0.08% - 0.17%), respectively. Cases with significant fibrosis accounted for 51.6% (33.4%-69.9%) in men and 34.4% (17.3%-51.4%) in women. There was no correlation between the HCV prevalence and age. Blood transfusion prior to 1992 strongly predicted significant fibrosis as did the history of injecting drug use (IDU) and ever having an HCV-infected sexual partner in men and caesarean sections in women. Factors associated with HCV infection without significant fibrosis were tattooing in men and younger age in women. We acknowledge limited possibility to study the associations between IDU and ever having HCV-infected sexual partner, given small sample sizes for these exposures.
Conclusions: As no clear birth cohort affected by HCV could be identified, risk factor-based screening in the general population should be considered, taking into account the association between the increased risk of liver fibrosis and the history of transfusion prior to 1992 and caesarean sections.
Conflict of interest statement
Figures
References
-
- Hatzakis A, Chulanov V, Gadano AC, Bergin C, Ben-Ari Z, Mossong J, et al. The present and future disease burden of hepatitis C virus (HCV) infections with today’s treatment paradigm—volume 2. J Viral Hepat. 2015;22: 26–45. doi: 10.1111/jvh.12351 - DOI - PubMed
-
- Perz JF, Armstrong GL, Farrington LA, Hutin YJF, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45: 529–538. doi: 10.1016/j.jhep.2006.05.013 - DOI - PubMed
-
- Wedemeyer H, Duberg AS, Buti M, Rosenberg WM, Frankova S, Esmat G, et al. Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepat. 2014;21: 60–89. doi: 10.1111/jvh.12249 - DOI - PubMed
-
- Foster GR, Irving WL, Cheung MCM, Walker AJ, Hudson BE, Verma S, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64: 1224–1231. doi: 10.1016/j.jhep.2016.01.029 - DOI - PubMed
-
- Sulkowski MS, Vargas HE, Di Bisceglie AM, Kuo A, Reddy KR, Lim JK, et al. Effectiveness of Simeprevir Plus Sofosbuvir, With or Without Ribavirin, in Real-World Patients With HCV Genotype 1 Infection. Gastroenterology. 2016;150: 419–429. doi: 10.1053/j.gastro.2015.10.013 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

