Plant flavones enhance antimicrobial activity of respiratory epithelial cell secretions against Pseudomonas aeruginosa
- PMID: 28931063
- PMCID: PMC5607194
- DOI: 10.1371/journal.pone.0185203
Plant flavones enhance antimicrobial activity of respiratory epithelial cell secretions against Pseudomonas aeruginosa
Abstract
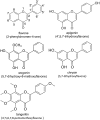
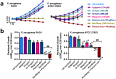
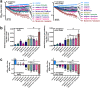
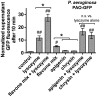
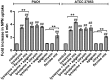
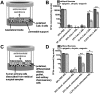
Flavones are a class of natural plant secondary metabolites that have anti-inflammatory and anti-bacterial effects. Some flavones also activate the T2R14 bitter taste receptor, which is expressed in motile cilia of the sinonasal epithelium and activates innate immune nitric oxide (NO) production. Flavones may thus be potential therapeutics for respiratory infections. Our objective was to examine the anti-microbial effects of flavones on the common sinonasal pathogens Candida albicans, Staphylococcus aureus, and Pseudomonas aeruginosa, evaluating both planktonic and biofilm growth. Flavones had only very low-level antibacterial activity alone. They did not reduce biofilm formation, but did reduce production of the important P. aeruginosa inflammatory mediator and ciliotoxin pyocyanin. However, flavones exhibited synergy against P. aeruginosa in the presence of antibiotics or recombinant human lysozyme. They also enhanced the efficacy of antimicrobials secreted by cultured and primary human airway cells grown at air-liquid interface. This suggests that flavones may have anti-gram-negative potential as topical therapeutics when combined with antibiotics or in the context of innate antimicrobials secreted by the respiratory or other epithelia. This may have an additive effect when combined with T2R14-activated NO production. Additional studies are necessary to understand which flavone compounds or mixtures are the most efficacious.
Conflict of interest statement
Figures

Similar articles
-
Flavones modulate respiratory epithelial innate immunity: Anti-inflammatory effects and activation of the T2R14 receptor.J Biol Chem. 2017 May 19;292(20):8484-8497. doi: 10.1074/jbc.M116.771949. Epub 2017 Apr 3. J Biol Chem. 2017. PMID: 28373278 Free PMC article.
-
Activation of airway epithelial bitter taste receptors by Pseudomonas aeruginosa quinolones modulates calcium, cyclic-AMP, and nitric oxide signaling.J Biol Chem. 2018 Jun 22;293(25):9824-9840. doi: 10.1074/jbc.RA117.001005. Epub 2018 May 10. J Biol Chem. 2018. PMID: 29748385 Free PMC article.
-
Synthesis and antibacterial activity of substituted flavones, 4-thioflavones and 4-iminoflavones.Bioorg Med Chem. 2006 Jul 15;14(14):4704-11. doi: 10.1016/j.bmc.2006.03.031. Epub 2006 Apr 5. Bioorg Med Chem. 2006. PMID: 16603364
-
Microbicidal activity of MDI-P against Candida albicans, Staphylococcus aureus, Pseudomonas aeruginosa, and Legionella pneumophila.Am J Infect Control. 2000 Jun;28(3):251-7. doi: 10.1067/mic.2000.105287. Am J Infect Control. 2000. PMID: 10840346 Review.
-
Plant antimicrobial agents and their effects on plant and human pathogens.Int J Mol Sci. 2009 Jul 31;10(8):3400-3419. doi: 10.3390/ijms10083400. Int J Mol Sci. 2009. PMID: 20111686 Free PMC article. Review.
Cited by
-
Efficacy and Mechanisms of Flavonoids against the Emerging Opportunistic Nontuberculous Mycobacteria.Antibiotics (Basel). 2020 Jul 27;9(8):450. doi: 10.3390/antibiotics9080450. Antibiotics (Basel). 2020. PMID: 32726972 Free PMC article. Review.
-
Distinct Cell Types With the Bitter Receptor Tas2r126 in Different Compartments of the Stomach.Front Physiol. 2020 Feb 7;11:32. doi: 10.3389/fphys.2020.00032. eCollection 2020. Front Physiol. 2020. PMID: 32116750 Free PMC article.
-
The effect of nano/microparticles of bee pollen on the shelf life of high-fat cooked sausage during refrigerated storage.Food Sci Nutr. 2024 Mar 7;12(6):4269-4283. doi: 10.1002/fsn3.4086. eCollection 2024 Jun. Food Sci Nutr. 2024. PMID: 38873449 Free PMC article.
-
The role of bitter and sweet taste receptors in upper airway innate immunity: Recent advances and future directions.World J Otorhinolaryngol Head Neck Surg. 2018 Aug 24;4(3):200-208. doi: 10.1016/j.wjorl.2018.07.003. eCollection 2018 Sep. World J Otorhinolaryngol Head Neck Surg. 2018. PMID: 30506052 Free PMC article. Review.
-
PAR-2-activated secretion by airway gland serous cells: role for CFTR and inhibition by Pseudomonas aeruginosa.Am J Physiol Lung Cell Mol Physiol. 2021 May 1;320(5):L845-L879. doi: 10.1152/ajplung.00411.2020. Epub 2021 Mar 3. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 33655758 Free PMC article.
References
-
- Stevens WW, Lee RJ, Schleimer RP, Cohen NA. Chronic rhinosinusitis pathogenesis. J Allergy Clin Immunol. 2015;136(6):1442–53. doi: 10.1016/j.jaci.2015.10.009 . - DOI - PMC - PubMed
-
- Lee RJ, Cohen NA. Role of the bitter taste receptor T2R38 in upper respiratory infection and chronic rhinosinusitis. Curr Opin Allergy Clin Immunol. 2015;15(1):14–20. doi: 10.1097/ACI.0000000000000120 - DOI - PMC - PubMed
-
- Hariri BM, Cohen NA. New insights into upper airway innate immunity. Am J Rhinol Allergy. 2016;30(5):319–23. doi: 10.2500/ajra.2016.30.4360 . - DOI - PMC - PubMed
-
- Alanis AJ. Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res. 2005;36(6):697–705. Epub 2005/10/12. doi: 10.1016/j.arcmed.2005.06.009 - DOI - PubMed
-
- Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2000 summary. Adv Data. 2002;(328):1–32. Epub 2003/03/29. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

