ERK1/2 signalling protects against apoptosis following endoplasmic reticulum stress but cannot provide long-term protection against BAX/BAK-independent cell death
- PMID: 28931068
- PMCID: PMC5607168
- DOI: 10.1371/journal.pone.0184907
ERK1/2 signalling protects against apoptosis following endoplasmic reticulum stress but cannot provide long-term protection against BAX/BAK-independent cell death
Abstract
Disruption of protein folding in the endoplasmic reticulum (ER) causes ER stress. Activation of the unfolded protein response (UPR) acts to restore protein homeostasis or, if ER stress is severe or persistent, drive apoptosis, which is thought to proceed through the cell intrinsic, mitochondrial pathway. Indeed, cells that lack the key executioner proteins BAX and BAK are protected from ER stress-induced apoptosis. Here we show that chronic ER stress causes the progressive inhibition of the extracellular signal-regulated kinase (ERK1/2) signalling pathway. This is causally related to ER stress since reactivation of ERK1/2 can protect cells from ER stress-induced apoptosis whilst ERK1/2 pathway inhibition sensitises cells to ER stress. Furthermore, cancer cell lines harbouring constitutively active BRAFV600E are addicted to ERK1/2 signalling for protection against ER stress-induced cell death. ERK1/2 signalling normally represses the pro-death proteins BIM, BMF and PUMA and it has been proposed that ER stress induces BIM-dependent cell death. We found no evidence that ER stress increased the expression of these proteins; furthermore, BIM was not required for ER stress-induced death. Rather, ER stress caused the PERK-dependent inhibition of cap-dependent mRNA translation and the progressive loss of pro-survival proteins including BCL2, BCLXL and MCL1. Despite these observations, neither ERK1/2 activation nor loss of BAX/BAK could confer long-term clonogenic survival to cells exposed to ER stress. Thus, ER stress induces cell death by at least two biochemically and genetically distinct pathways: a classical BAX/BAK-dependent apoptotic response that can be inhibited by ERK1/2 signalling and an alternative ERK1/2- and BAX/BAK-independent cell death pathway.
Conflict of interest statement
Figures
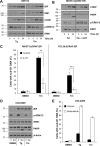
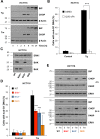
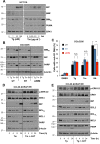
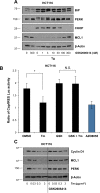
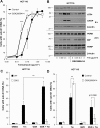
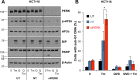
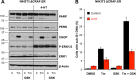
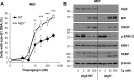
Similar articles
-
Suppression of PI3K/Akt signaling by synthetic bichalcone analog TSWU-CD4 induces ER stress- and Bax/Bak-mediated apoptosis of cancer cells.Apoptosis. 2014 Nov;19(11):1637-53. doi: 10.1007/s10495-014-1031-y. Apoptosis. 2014. PMID: 25183449
-
ROS mediated ER stress induces Bax-Bak dependent and independent apoptosis in response to Thioridazine.Biomed Pharmacother. 2018 Oct;106:200-209. doi: 10.1016/j.biopha.2018.06.123. Epub 2018 Jun 28. Biomed Pharmacother. 2018. PMID: 29960166
-
Coumestrol induces mitochondrial dysfunction by stimulating ROS production and calcium ion influx into mitochondria in human placental choriocarcinoma cells.Mol Hum Reprod. 2017 Nov 1;23(11):786-802. doi: 10.1093/molehr/gax052. Mol Hum Reprod. 2017. PMID: 29040664
-
Endoplasmic reticulum-mediated unfolded protein response and mitochondrial apoptosis in cancer.Biochim Biophys Acta Rev Cancer. 2017 Jan;1867(1):58-66. doi: 10.1016/j.bbcan.2016.12.002. Epub 2016 Dec 15. Biochim Biophys Acta Rev Cancer. 2017. PMID: 27988298 Free PMC article. Review.
-
The role of MAPK signalling pathways in the response to endoplasmic reticulum stress.Biochim Biophys Acta. 2014 Oct;1843(10):2150-63. doi: 10.1016/j.bbamcr.2014.01.009. Epub 2014 Jan 15. Biochim Biophys Acta. 2014. PMID: 24440275 Review.
Cited by
-
Splice switching an oncogenic ratio of SmgGDS isoforms as a strategy to diminish malignancy.Proc Natl Acad Sci U S A. 2020 Feb 18;117(7):3627-3636. doi: 10.1073/pnas.1914153117. Epub 2020 Feb 4. Proc Natl Acad Sci U S A. 2020. PMID: 32019878 Free PMC article.
-
Hsa_circ_0002348 regulates trophoblast proliferation and apoptosis through miR-126-3p/BAK1 axis in preeclampsia.J Transl Med. 2023 Jul 28;21(1):509. doi: 10.1186/s12967-023-04240-1. J Transl Med. 2023. PMID: 37507742 Free PMC article.
-
Endoplasmic reticulum stress contributes to the pathogenesis of stress urinary incontinence in postmenopausal women.J Int Med Res. 2018 Dec;46(12):5269-5277. doi: 10.1177/0300060518807602. Epub 2018 Nov 14. J Int Med Res. 2018. PMID: 30426803 Free PMC article.
-
Edaravone Ameliorates Renal Warm Ischemia-Reperfusion Injury by Downregulating Endoplasmic Reticulum Stress in a Rat Resuscitation Model.Drug Des Devel Ther. 2020 Jan 15;14:175-183. doi: 10.2147/DDDT.S211906. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32021102 Free PMC article.
-
Investigation the apoptotic effect of silver nanoparticles (Ag-NPs) on MDA-MB 231 breast cancer epithelial cells via signaling pathways.Heliyon. 2024 Feb 26;10(5):e26959. doi: 10.1016/j.heliyon.2024.e26959. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38455550 Free PMC article.
References
-
- Ron D & Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2011; 8: 519–529 - PubMed
-
- Walter P & Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011; 334: 1081–1086 doi: 10.1126/science.1209038 - DOI - PubMed
-
- Gardner BM & Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science. 2011; 333: 1891–1894 doi: 10.1126/science.1209126 - DOI - PMC - PubMed
-
- Harding HP, Zhang Y & Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999; 397: 271–274 doi: 10.1038/16729 - DOI - PubMed
-
- Harding HP, Zhang Y, Bertolotti A, Zeng H and Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000; 5: 897–904 - PubMed
MeSH terms
Substances
Grants and funding
- BBS/E/B/000C0413/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 14867/CRUK_/Cancer Research UK/United Kingdom
- BBS/E/B/000C0419/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/E02162X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/B/000C0417/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

