Prevalence of Human Papillomavirus Among Females After Vaccine Introduction-National Health and Nutrition Examination Survey, United States, 2003-2014
- PMID: 28931217
- PMCID: PMC5740482
- DOI: 10.1093/infdis/jix244
Prevalence of Human Papillomavirus Among Females After Vaccine Introduction-National Health and Nutrition Examination Survey, United States, 2003-2014
Abstract
Background: Human papillomavirus (HPV) vaccine was recommended in 2006 for routine vaccination of US females aged 11-12 years. Most vaccine used through 2014 was quadrivalent vaccine (4vHPV), which prevents HPV-6, -11, -16, and -18 infection. To evaluate vaccine impact, we measured HPV prevalence in the National Health and Nutrition Examination Survey (NHANES).
Methods: We analyzed HPV DNA types detected in self-collected cervicovaginal specimens and demographic, sexual behavior, and self-reported vaccination data from females 14-34 years old. We estimated HPV prevalence in the prevaccine (2003-2006) and vaccine eras (2007-2010 and 2011-2014).
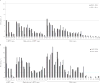
Results: Among 14- to 19-year-olds, 4vHPV-type prevalence decreased from 11.5% (95% confidence interval [CI], 9.1%-14.4%) in 2003-2006 to 3.3% (95% CI, 1.9%-5.8%) in 2011-2014, when ≥1-dose coverage was 55%. Among 20- to 24-year-olds, prevalence decreased from 18.5% (95% CI, 14.9%-22.8%) in 2003-2006 to 7.2% (95% CI, 4.7%-11.1%) in 2011-2014, when ≥1-dose coverage was 43%. Compared to 2003-2006, 4vHPV prevalence in sexually active 14- to 24-year-olds in 2011-2014 decreased 89% among those vaccinated and 34% among those unvaccinated. Vaccine effectiveness was 83%.
Conclusions: Within 8 years of vaccine introduction, 4vHPV-type prevalence decreased 71% among 14- to 19-year-olds and 61% among 20- to 24-year-olds. Estimated vaccine effectiveness was high. The decrease in 4vHPV-type prevalence among unvaccinated females suggests herd protection.
Keywords: HPV vaccine; human papillomavirus; prevalence; vaccine effectiveness; vaccine impact.
Published by Oxford University Press for the Infectious Diseases Society of America 2017. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Conflict of interest statement
Figures

References
-
- Markowitz LE, Dunne EF, Saraiya M, et al. Centers for Disease Control and Prevention (CDC) Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2014;63:1–30. - PubMed
-
- Centers for Disease Control and Prevention (CDC) Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1705–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

